IN THE LAB TODAY 002: HOW CHEMICAL CHLORINE TRIFLUORIDE CAN MAKE ANYTHING BURST INTO FIRE ON CONTACT WITH ASBESTOS.
A little bit of danger can be so exciting at times, that’s when they are not as much as what cannot be controlled. You will want to ask me about this chemical so let’s go;
Chlorine Trifluoride is an interhalogen compound with the formula CIF³. This chemical is in liquid form, it’s poisonous, corrosive and colorless. This chemical is so corrosive that it can make practically anything burst into flames on contact, and extremely reactive gas condenses to a pale-greenish yellow liquid, the form in which it is most often sold.
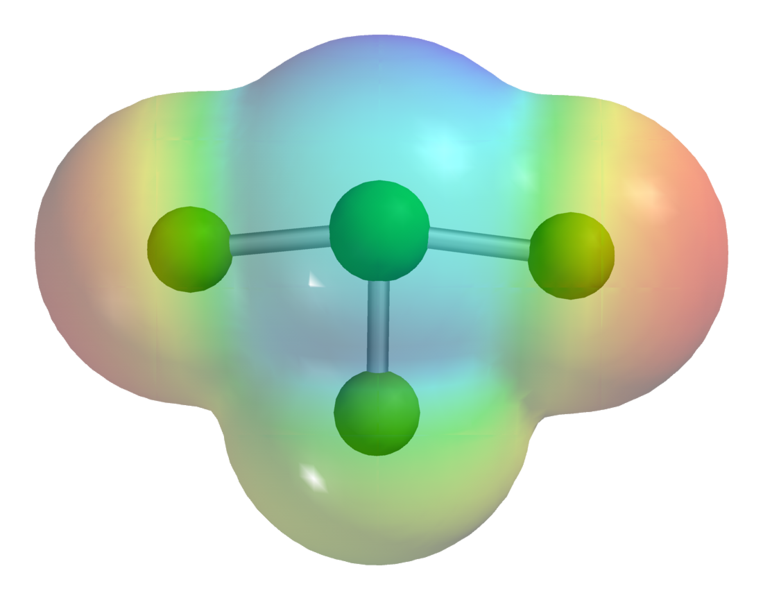
Chlorine Trifluoride on m.common.wikimedia.org
Formula: CIF3
Molar mass: 92.448g/mol
Boiling Point: 11.75° C
Common Name: N-Stoff
As of fluorine, it is the most powerful element of the oxidizing agents out of all the elements, in fact, with its strong oxidizing abilities. It is so reactive that it oxidizes water to oxygen and many metal oxides to metal halides on contacts.
ClF3+ 2H2O → 3HF + HCl + O2
ClF3+ H2O → HF + HCl + OF2
As I have said, this chemical is a very strong oxidizing and fluorinating agent which reacts with the highest inorganic and organic materials such as, glass, ceramics, and metals. It is very hazardous, because of it’s highly flammable materials which are without any ignition source. That is why it’s very important to take note of any equipment that will come into contacts with this chemical, such equipment must be passivated and scrupulously cleaned before coming into contacts with chlorine trifluoride.
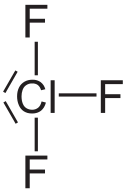
Structure of Chlorine trifluoride
In World War II, this chemical was discovered by a scientist called Nazis who took interest in using the chemical in as a means of fighting enemies then, although this chemical is not later put to use because the weapon was incredibly dangerous to those carrying around and those they launch it too.
However, Nazir was successful in creating tons of this chemicals to form weapons in a secret facility known as Falkenhagen Bunker before the chemical was captured by the Russians Military in 1944.
You don’t really have to worry about this weapon perhaps you’re thinking it can still be used. No. the compound has been banned under the Chemical Weapons Convention (CWC) after the war.
File:90mm Anti-Aircraft Gun, USA, World War II, view 1 - National Electronics Museum - DSC00612.JPG
Reason has been that, as chlorine trifluoride is an oxidizing agent, it has been reported to ignite sand, asbestos and other highly fire-retardant materials and it will also ignite the ashes of materials that have already been burned in oxygen.
Let’s get back to what oxidizing agents really is,
In chemistry, oxidizing agents is a substance that has the ability to oxidize other substance to cause them to lose electrons. Some common oxidizing agents are oxygen, hydrogen peroxide, and the halogens.
However, this same chemical also has its usefulness;
In Semiconductor Industry.
Its use in Semiconductor Industry, to clean chemical vapor deposition chambers.
- It can be used to remove semiconductor material from the chamber walls without the need to dismantle the chamber.
- It does not need to be activated by the use of plasma since the heat of the chamber is enough to decompose and react with the semiconductor material.
In Rocket Propellant
Chemical Chlorine trifluoride is said to have been investigated to as a high-performance storable oxidizer in rocket propellant system.
Effects of Chlorine Fluoride
When chloride trifluoride comes into contact with water, it will react explosively with it and as a fun byproduct creates large amounts of dangerous gasses such as hydrofluoric acid and hydrochloric acid.
Hydrochloric acid, in particular, is incredibly dangerous and along with being able to melt things like glass and concrete, can permanently damage your lungs and eyes. As if that wasn’t worrying enough, if you’re ever unlucky enough to get hydrofluoric acid on your skin, it doesn’t actually hurt until a few hours later. After it has absorbed a bit, it starts destroying your nerves and bones and can ultimately cause cardiac arrest when it gets into your bloodstream.
In 1994, a lab technician in Australia accidentally spilled hydrofluoric acid on his lap and despite immediately executing safety procedures including hosing off, immersing himself in a swimming pool and later extensive medical care including needing to have one of his leg amputated, he was reported dead two weeks of the accident.
Below are the type Of Hazards/Exposure-Prevention and First Aids Treatment.
.jpg)
File:Caution (3928133058).jpg
This image was originally posted to Flickr
| HAZARD/EXPOSURE | SYMPTOM | PREVENTION | TREATMENT |
|---|---|---|---|
| FIRE | Gives off highly toxic fumes in a fire, Many reactions may cause explosion | NO contact with water and any flammable substances. | In case of fire in the surroundings: NO hydrous agents. |
| SKIN | Redness. Serious skin burns. Pain. Blisters | Protective gloves. Protective clothing | First rinse the contaminated skin with water. If this chemical penetrates the clothing, immediately remove the clothing and flush the skin with water. Get medical attention promptly. |
| EYES | Redness. Pain. Severe deep burns. Permanent loss of vision. | Face shield or eye protection in combination with breathing protection. | First rinse with plenty of water for several minutes, occasionally lifting the lower and upper lids. Get medical attention immediately.PS: Contact lenses should not be worn when working with this chemical. |
| INHALATION | Burning sensation. A cough. A sore throat. Labored breathing. Shortness of breath. | Ventilation, local exhaust, or breathing protection. | Fresh air, rest. Half-upright position. Refer for medical attention |
There are a couple other useful applications though. For instance, it’s great for plasma less cleaning of certain surfaces used in semiconductor manufacturing and it also works well at cleaning uranium residue off of the walls of nuclear power plants and removing built up oxides.
If you liked this article, show some love
References;
Chlorine Trifluoride
Chlorine Trifluoride²
Chlorine Trifluoride - Compound Summary
Nazir Interest In CIF3 In World War II
This is an interesting piece that I thoroughly enjoyed @bollutech!
I finally know what makes Hydrochloric acid—which is already extremely dangerous. Chlorine Trifluoride is like an instant fire. If someone were to pour some on glass, would it melt???
If you find this post very interesting , you can get it to friend and follower. Thanks @shello
Sorry @bollutech,
I ended up not sharing it, because I don't understand the post too much myself. I hope you can forgive me!
With love,
@shello
Hello @shello ,
The chemical chlorine trifluoride can't melt glass it can only set fire to glass but it's extremely toxic, although, that’s the least of the problem. It is hypergolic with every known fuel, and so rapidly hypergolic that no ignition delay has ever been measured.
It is also hypergolic with such things as cloth, wood, and test engineers, not to mention asbestos, sand, and water-with which it reacts explosively."
However, this chemical can only be kept in some of the ordinary structural metals-steel, copper, aluminum, etc.-because of the formation of a thin film of insoluble metal fluoride which protects the bulk of the metal, just as the invisible coat of oxide on aluminum keeps it from burning up in the atmosphere. If, however, this coat is melted or scrubbed off, and has no chance to reform, the operator is confronted with the problem of coping with a metal-fluorine fire.
Therefore, for dealing with this situation, I have always recommended a good pair of running shoes.
Much love
@bollutech
Hahaha, note taken. That's interesting that something so hazardous can be held by normal metals. Still not going to touch or breathe it though!~
Nice photo of the "flak" (AA-gun)
And the molecule looks unstable even as the formula :)
Thank you for your comment, it has been checked and corrected.
Thanks for notifying me.
Hi LOVE YOUR POST MAN!!! LIKE MY POST TOO!!!!! HERE IS THE LINK: https://steemit.com/bots/@abusereports/last-minute-upvote-list-2018-05-02