Searching For The Elusive Metallic Hydrogen
In 2016, a team of scientists claimed they to have finally solved the 1935 problem by creating the metallic hydrogen which was equivalent to the holy grail of high-pressure physics.
This idea of all matter capable of turning into a metal under high enough pressure was first put forward in 1926 by John Desmond Bernal, an Irish scientist.
On becoming metallic, the matter will have an abundance of free electrons capable of conducting electricity very efficiently.

[Flickr]CC BY 2.0 Authored by Robert Couse-Baker: A Metallic Hydrogen claimed to be produced in a laboratory in October 2016 at a pressure of about 495 GPa. Finding yet to be replicated
In 1935, the two physicists Eugene Wigner and Hillard Bell Huntington, with the former winning a Nobel Prize in physics in 1963, started investigating the possibility of making a hydrogen atom behave like an alkali metal under a proposed high pressure of at least 25,000 atmospheres or 25GPa.
The idea was that it is possible to produce metallic hydrogen under very high pressure, which forms like the diamond, which will remain stable even under lower pressure.
Why Do The World Need a Metallic Hydrogen?
The doubt and drama that followed the 2016 report of metallic hydrogen will get anyone inquisitive enough to ask, "What is the big deal with metal hydrogen?"
Up until the claim, hydrogen metal was predicted only theoretically with no real production of one in a laboratory.
A Little on Hydrogen
Hydrogen is the first element on the periodic table, one of the most abundant elements in nature. It occurs in most places as a gas. Reducing the temperature of hydrogen low enough turns it into a liquid. Why go to all the troubles? The answer is simply because it makes an excellent rocket fuel. Hydrogen in liquid form is one of the best rocket fuel that is obtainable. It is light, and it yields a more rapid burn of extreme intensity.
When the liquid hydrogen is mixed with an oxidant (dissolved oxygen), it produces the highest efficient rocket fuel capable of creating the highest specific impulse. Specific impulse is the time in seconds that a thrust of a kilogram of force can be maintained by a kilogram mass of fuel.
The liquid hydrogen-oxygen combo reaction:
It creates about 10 megajoules per kilogram (MJ/kg) of energy in the space shuttle. That is a lot of energy released when you realise that TNT produces only 4.2 MJ/kg of energy.
Looking into this further, the hydrogen and oxygen in a water molecule have the hydrogen existing as two molecules of the same element (diatomic element)- H2 with Oxygen existing as diatomic two (O2.
Each hydrogen molecule and oxygen bond have its bond broken and reassembled to produce the net energy release of 10MJ/kg.
If the metastability of the metallic hydrogen is suppressed, there will be a recombination of atoms of hydrogen molecules. The recombination would result in an energy release of 216 MJ/kg-this is greater than 20 times the energy gotten( which is 10 MJ/kg) in Space Shuttle combustion engine using hydrogen and oxygen.
The production of metallic hydrogen will, therefore, yield the most powerful potential source of rocket fuel.
This energy produced which is the equivalent of 50 TNTs is not the only the excellent feature of the metallic hydrogen, the exhaust that would be the lightest product since it will be hydrogen gas crowns the metallic hydrogen king of space exploration fuel.
But like most offers that might sound too good to be true, there is a catch here. Hydrogen element is a bit clingy; they find it hard to exist by themselves. It is a diatomic element, they will combine willingly with atoms of same element to be stable.
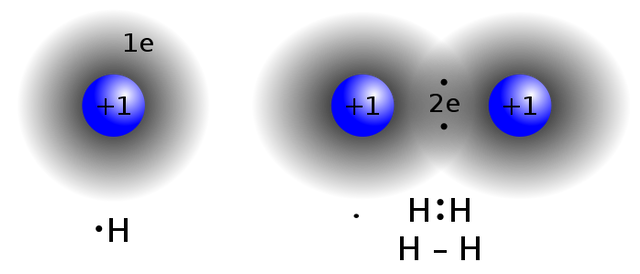
[Wikipedia]Creative Commons Attribution-Share Alike 3.0 by Jacek FH
H. + H. → H : H = H- H = H2
Each of the hydrogen atoms acquires a stable outer shell of two electrons (2e).
Remember the single hydrogen atom has one electron that is free. The valence 1 electrons are highly unstable. It is either they take 1 electron from the adjacent hydrogen atom to fill up its orbital, or they give up one.
Through the sharing of the two electrons, they form a hydrogen bond- the two H atom will become more stable than a single atom.
Making a metallic hydrogen
Theoretically, hydrogen can be metallic under some extreme pressure and circumstance. This freaky circumstance is believed to be met in the core of Jupiter under tremendous pressure of about 5000 bars.
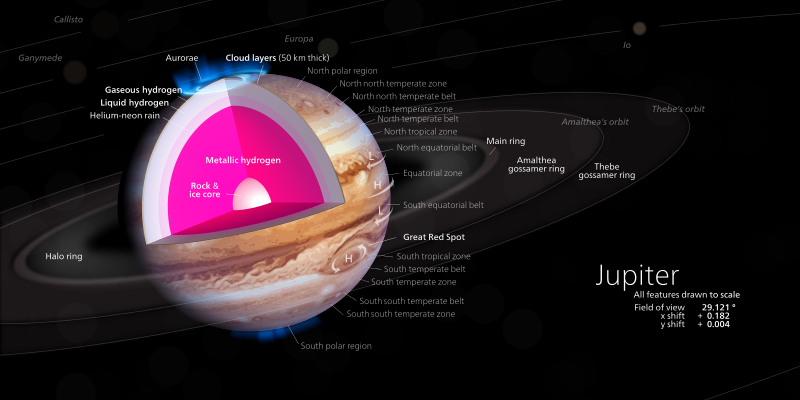
[Wikipedia]Creative Commons Attribution-Share Alike 3.0 by Bammesk: Scientists are not sure if Jupiter has such a thing as metallic hydrogen, it is purely theoretical at the moment
The Jupiter is made up of 90% hydrogen and deep down it, the tremendous pressure of the Jupiter core as a result of its massive size, squeezes the electrons out of the hydrogen atoms and it changes it into a metal. Like all metals, it then becomes a conductor, not just any conductor but a perfect conductor (superconductor).
Making a Metallic Hydrogen
In the past, the researchers tried to make metallic hydrogen same way olden day alchemists attempted to transform a standard metal into a valuable precious metal.
The various process which include but not limited to squeezing hydrogen atoms until it gives up its electrons, using electric field through a device called the Z machine to transform hydrogen into metal, using a high velocity light-gas gun to create shock pressure to metalise hydrogen. None of the processes produced a stable hydrogen metal that is usable once the pressure is no longer there.
But in October 2016, two physicists made an announcement that they can produce hydrogen metal through the use of a diamond anvil at high pressure while working at very low temperature.
Observing the hydrogen on the diamond anvil, the two scientists from Harvard Isaac Silvera and Ranga Dias increased the pressure at a temperature slightly above absolute zero (5.5 Kelvin or - 450-degree Fahrenheit). After some time the once transparent hydrogen turned black. Finally, at 495 GPa or approximately 5 million times Earth's atmospheric pressure, the hydrogen becomes reflective. The researchers have this as proof of the hydrogen having changed its atom structure into a 3-D structure such as obtainable from a metal.
The Backclash
An expert in the diamond-anvil cell (DAC) Eugene Gregoryanz from The University of Edinburgh belived the changes observed in reflectivity are too big for real hydrogen.
"The word garbage cannot really describe it," says Eugene Gregoryanz, a high-pressure physicist at the University of Edinburgh, who objects to several of the experiment's procedures.Sciencemag
He is not the only scientists who faulted the claim. Mikhail Eremets working on solid metallic hydrogen in Germany said the result was not convincing.
A French physicist, Paul Loubeyre informed Nature of his doubt on the purported pressure used.
Conclusion
The researchers did not help matters too as they lost the sample as they wanted to do further tests.
So now we may never know what the situation was, but whatever it is, it would be nice to have hydrogen metal here
to understand more the behaviour of planets like Jupiter and Saturn-thought to have hydrogen as a metal. Its use as a rocket fuel with massive energy release will then be a plus.
- A Breakthrough in High Pressure Physics?
- Stability of Hydrogen at Ambient Condition
- 1997 Nature Article News: Will solid hydrogen
ever be a metal? - Jan 2017 Nature Article: Physicists doubt bold report of metallic hydrogen
- NASA: Liquid Hydrogen-The Fuel of Choice for Space Exploration
- Metallic Hydrogen Claim Faces Fierce Scrutiny
- Jupiter: The Home of Hydrogen Metal
Interesting read. Long though, but was worth the time. Good job. Keep it up.
I appreciate the encouragement. Thank you.
A scientific experiment has to be able to be replicated. I'm sure this method will be tried again, by them or other people to see if they get any results and whether they can improve the method.
Except for the rocket fuel, metallic hydrogen would be useful as a room-temperature superconductor, transmitting electrical power anywhere in the world without losing any power. But this is still very hypothetical.
That is very correct, if we cannot replicate a given method with others getting similar result, it cannot be said to be a breakthrough. Thank you.