Find an unknown concentration by conductimetry (my actual results)
Introduction
An assay, or a titration is a consistent method searched for the amount of material of a chemical species dissolved in solution.
-Reactive titrant:
This is the chemical species whose concentration is known
-Reactive titrated:
It is the chemical species of which one wants to know the quantity of the matter or the concentration.
-equivalence:
Equivalence is achieved when the reactants are mixed in the stoichiometric proportions of the equation of the titration reaction.
Goal :
Determination of the unknown concentration of a strong acid (HCl) neutralized with a strong base (NaOH) by means of a conductivity meter.
Principle:
The evolution of an acid-base titration can be followed by changes in conductivity during the titration.
Materials and products used:
Beaker, pro pipette, Burette, Magnetic stirrer, Magnetic stirrer, Conductivity meter, Erlenmeyer flask, Distilled water, NaOH solution, HCl solution.




.jpg)
.jpg)

Operating mode:
take 20 ml of a hydrochloric acid solution of unknown concentration by means of a pipette using the pro-pipette pear. Then transfer this volume taken from an Erlenmeyer flask containing a magnetic bar to allow magnetic stirring, otherwise homogenization is obtained by stirring after each addition of reagent and before each point. To do this, fill a burette graduated with 0.2N sodium hydroxide solution. start titrating gently, with magnetic or manual stirring, after each 0.5ml volume of the NaOH poured, by opening the burette tap slightly while checking the measured conductivity value at a conductivity meter whose electrodes are immersed in the solution a analyze contained in the Erlenmeyer flask.
Exploitation of results :
1-Dosing Scheme:
NaOH+HCl==NaCl+H2O
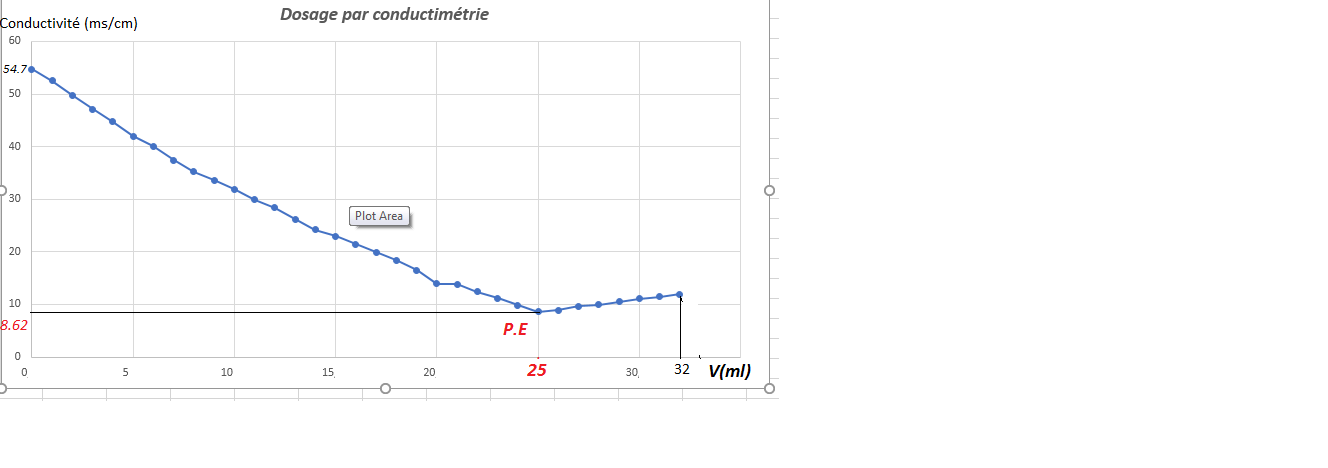
2-The Graph:
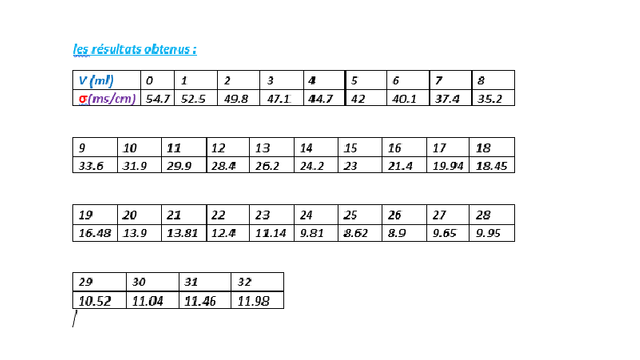
The obtained results :
-The acid-base titration can be followed through the variation of the conductivity.
-Be noted that the conductivity decreases to the equivalence point (P.E) because the H + ions of the acid are replaced by Na + less mobile ions. But on the contrary, it increases in the presence of an excess of sodium hydroxide NaOH at the point of equivalence since new ions Na + and OH- are introduced in solution.
3-The equivalence point:
a 25 ml of NaOH we notice the turn in the graph where the conductivity increases VE = 25ml and σ = 8.62ms / cm
4-Calculation of HCl concentration:
We have: CHcl. VHcl = CNaoH. VNaoH
CHCl = (CNaoH, VNaoH) / VHCl = (0.2.25) / 30 = 0.16N
CHCl = 0,16N
Conclusion:
The conductivity informs us about the amount of total ions in the solution, the more the liquid contains more ions the more it conducts the current.