Have you ever wondered how or why iron is oxidized ?, In this post, I will explain what happens. I hope you like!

Why is iron oxidized?
The oxidation of iron is due to a redox (or oxidation-reduction) reaction. This is a type of reaction in which they are exchanged electrons between the reagents so that they change their oxidation states. In every redox reaction there is a substance that loses electrons (it oxidizes) acting as a reducer and a substance that gains electrons (it is reduced) and it acts as an oxidant. A daily example of iron pieces exposed to the air and humidity. After a while seen as a layer of iron oxide is formed and we say that it has oxidized.
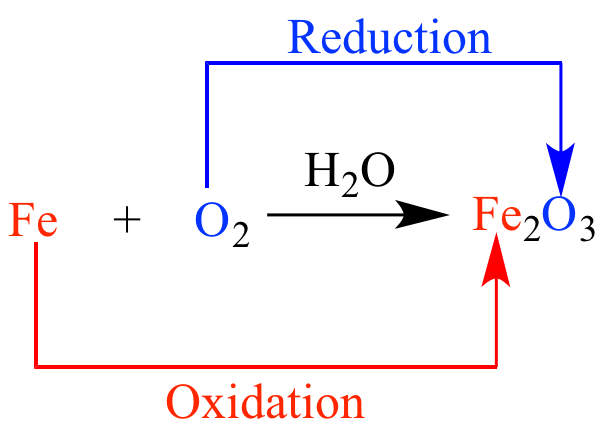 The Oxidation of Iron
The elements have a 0 oxidation state and thus iron can oxidize, losing electrons, which are transferred to oxygen, which gains two electrons and reduces, going from oxidation state 0 to -2. The reaction global could be summarized as: 4 Fe + 3 O2 ---> 2 Fe2 O3. As it is only required that there is electron transfer by an atom and gain of electrons by a different one, iron is also oxidized in an atmosphere of chlorine, sulfur or oxygen.
The Oxidation of Iron
The elements have a 0 oxidation state and thus iron can oxidize, losing electrons, which are transferred to oxygen, which gains two electrons and reduces, going from oxidation state 0 to -2. The reaction global could be summarized as: 4 Fe + 3 O2 ---> 2 Fe2 O3. As it is only required that there is electron transfer by an atom and gain of electrons by a different one, iron is also oxidized in an atmosphere of chlorine, sulfur or oxygen.
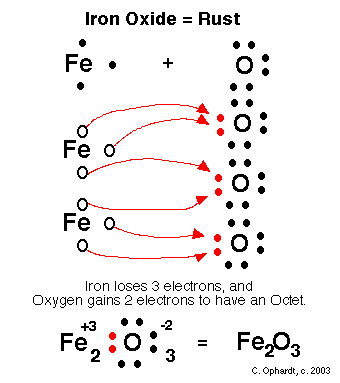
The Oxidation of Iron
How we may have seen, the reactions of oxide reduction which are known for centuries, are responsible of the oxidation of iron something that we can see in our everyday life.
If you liked this Post, upvote and reesteem!






Excellent post, interesting photos. Godspeed!