Separation of the Components of a Mixture
Separation of the Components of a Mixture
Objective: To become familiar with the methods of separating substances from one another using decantation, extraction and sublimation techniques.
Materials:
Apparatus
Balance bunsen burner and hose tongs evaporating dishes (2) watch glass
50 or 100 mL graduated cylinder clay triangles (2) or wire gauze (2) ring stands(2) iron rings(2) glass stirring rods
Chemicals:
Unknown mixture of sodium chloride, ammonium chloride and silicon dioxide
Procedures:
The mixture separated contained NaCl, NH₄Cl and SiO₂. Their separation was accomplished through subliming the NH₄Cl, extracting the NaCl with water and drying the remaining SiO₂.
The mass of an evaporating dish was carefully determined to the nearest 0.01 g and a 2-3 gram unknown mixture sample was obtained from the instructor while the number on the sample was recorded. The mass of the evaporating dish and the unknown sample was then recorded to the nearest 0.01g.
The evaporating dish containing the unknown sample was placed on a clay triangle and ring stand assembly located beneath a fume hood. The dish was then heated carefully to avoid splattering with a burner until white fumes no longer formed.
The evaporating dish was allowed to cool until it reached room temperature, only then was the mass of the dish and the remaining unknown substance recorded. Next, the lost mass was calculated and recorded.
25 mL of water was added into the unknown mixture and gently stirred for 5 minutes. Then the mass of another evaporating dish and a watch glass was determined. The liquid was decanted carefully, so as to not transfer any solid, into this second evaporating dish.
10 mL more water was added into the unknown mixture, containing solids, and subsequently decanted into the second evaporating dish. This step was repeated once more so that one dish contained wet sand and the other a salt water solution.
The evaporating dish with the salt solution was carefully placed on the clay triangle and the water gently evaporated to avoid splattering. When almost all the water had evaporated the watch glass was placed on top and the heat lowered to stop the salt from escaping.
When the water had completely evaporated the evaporating dish was left to cool and then the mass of the salt and the dish was recorded.
Meanwhile the wet sand in the original evaporating dish was placed in an oven and left overnight to dry out. The next day the mass of the sand and the dish was recorded.
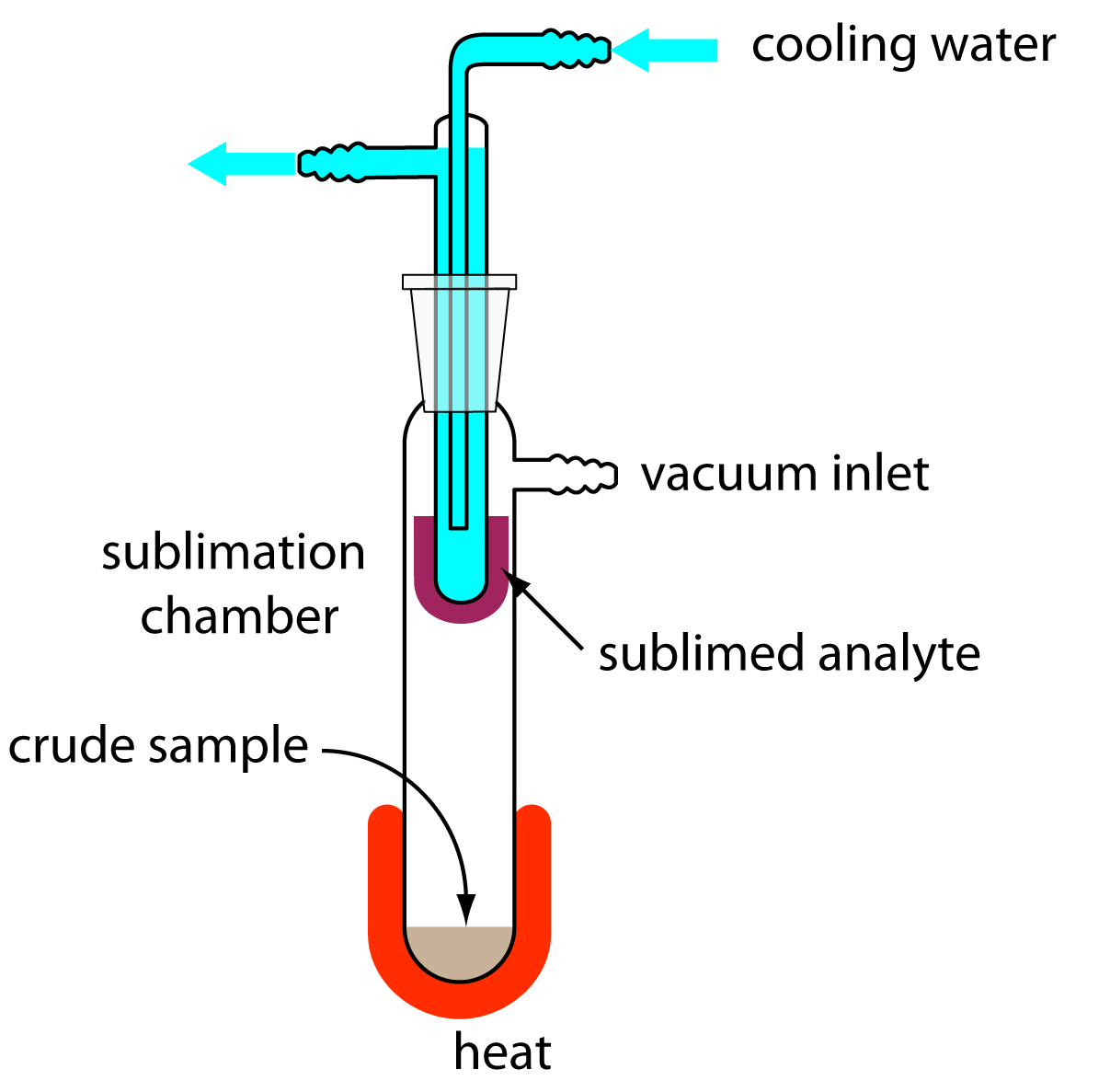
Observations/Data Analysis:
A. Mass of Evaporating Dish and Original Sample
49.74g
Mass of evaporating dish
46.27g
Mass of original sample
3.46g
Mass of evaporating dish after subliming NH4Cl
49.00g
Mass of NH4Cl
0.74g
Percent of NH4CL
21%
B. Mass of evaporating dish, watch glass, and NaCl
91.27g
Mass of evaporating dish and watch glass
90.50g
Mass of NaCl
0.77g
Percent of NaCl
22%
C. Mass of Evaporating Dish and SiO2
47.94g
Mass of evaporating dish
46.27g
Mass of SiO2
1.67g
Percent of SiO2
48.3%
D. Mass of Original Sample
3.46g
Mass of determined
3.18g
Differences in weight of original and determined
0.28g
Percent recovery of matter
91.9%
Calculations: all mass in grams
Mass of NH4Cl=49.74-49.00=0.74 (2 decimal places)
Percent of NH4Cl=0.74/3.46=21% (2 sig figs)
Mass of NaCl=91.27-90.50=0.77 (2 decimal places)
Percent of NaCl=0.77/3.46=22% (2 sig figs)
Mass of SiO2=47.94-46.27=1.67 (2 decimal places)
Percent of SiO2=1.67/3.46=48.3% (3 sig figs)
Mass of determined=0.74+0.77+1.67=3.18 (2 decimal places)
Differences in weight of original and determined=3.46-3.18=0.28 (2 decimal places)
Percent recovery of matter=3.18/3.46=91.9% (3 sig figs)
Account for errors: While decanting the salt water solution from the sand some water was spilt and possibly some sand poured into the second evaporating dish as well. Furthermore while evaporating the water from the salt the heat became too strong and the water spilt over, therefore salt and water were both lost. Therefore a sizeable amount of salt was lost and perhaps some sand misplaced with the salt.
Congratulations @asunarose! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPCongratulations @asunarose! You have received a personal award!
Click on the badge to view your Board of Honor.
Congratulations @asunarose! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!