Plant Nutrition
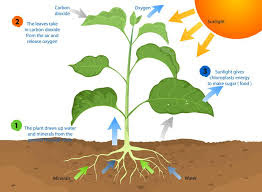
Plant Nutrition Plants need eighteen chemical elements for their growth—carbon (C), hydrogen (H), oxygen (O), nitrogen (N), phosphorus (P), potassium (K), sulfur (S), calcium (Ca), magnesium (Mg), iron (Fe), manganese (Mn), boron (B), zinc (Zn), molybdenum (Mo), nickel (Ni), copper (Cu), cobalt (Co), and chlorine (Cl). Plants obtain carbon as carbon dioxide (CO2) and oxygen partially as oxygen gas (O2) from the air. The remaining essential elements are obtained mainly from the soil. The avail-ability of these nutrients is influenced either directly or indirectly by the presence of organic matter. The elements needed in large amounts—carbon, hydrogen, oxygen, nitrogen, phosphorus, potassium, calcium, mag-nesium, sulfur—are called macronutrients. The other elements, called micronutrients, are essential elements needed in small amounts. (Sodium [Na] helps many plants grow better, but it is not considered essential to plant growth and reproduction.) Nutrients from decomposing organic matter. Most of the nutrients in soil organic matter can’t be used by plants as long as those nutrients exist as part of large organic molecules. As soil organisms decompose organic matter, nutrients are converted into simpler, inorganic, or mineral forms that plants can easily use. This process, called mineralization, provides much of the nitrogen that plants need by converting it from organic forms. For example, proteins are converted to ammonium (NH4+) and then to nitrate (NO3–). Most plants will take up the majority of their nitrogen from soils in the form of nitrate. The mineralization of organic matter is also an important mechanism for supplying plants with such nutrients as phosphorus and sulfur and most of the micronutrients. This release of nutrients from organic matter by mineralization is part of a larger agricultural nutrient cycle (see figure 2.4). For a more detailed discussion of nutrient cycles and how they function in various cropping systems, see chapter 7. Addition of nitrogen. Bacteria living in nodules on legume roots convert nitrogen from atmospheric gas (N2) to forms that the plant can use directly. A number of free-living bacteria also fix nitrogen. Storage of nutrients on soil organic matter. Decomposing organic matter can feed plants directly, but it also can indirectly benefit the nutrition of the plant. A number of essential nutrients occur in soils as positively charged molecules called cations (pronounced cat-eye-ons). The ability of organic matter to hold on to cations in a way that keeps them available to plants is known as cation exchange capacity (CEC). Humus has many negative charges. Because opposite charges attract, humus is able to hold on to positively charged nutrients, such as calcium (Ca++), potassium (K+), and magnesium (Mg++) (see figure 2.5a). This keeps them from leaching deep into the subsoil when water moves through the topsoil. Nutrients held in this way can be gradually released into the soil solution and made available to plants throughout the growing season. However, keep in mind that not all plant nutrients occur as cations. For example, the nitrate form of nitrogen is negatively charged (NO3–) and is actually repelled by the negatively charged CEC. Therefore, nitrate leaches easily as water moves down through the soil and beyond the root zone. Clay particles also have negative charges on their surfaces (figure 2.5b), but organic matter may be the major source of negative charges for coarse and medium-textured soils. Some types of clays, such as those found in the southeastern United States and in the tropics, tend to have low amounts of negative charge. When those clays are present, organic matter may be the major source of negative charges that bind nutrients, ven for fine-textured (high-clay-content) soils. Protection of nutrients by chelation. Organic molecules in the soil may also hold on to and protect certain nutrients. These particles, called “chelates” (pronounced key-lates) are by-products of the active decomposition of organic materials and are smaller than the particles that make up humus. In general, elements are held more strongly by chelates than by binding of positive and negative charges. Chelates work well because they bind the nutrient at more than one location on the organic molecule (figure 2.5c). In some soils, trace elements, such as iron, zinc, and manga-nese, would be converted to unavailable forms if they were not bound by chelates. It is not uncommon to find low-organic-matter soils or exposed subsoils deficient in these micronutrients. Other ways of maintaining available nutri-ents. There is some evidence that organic matter in the soil can inhibit the conversion of available phosphorus to forms that are unavailable to plants. One explanation is that organic matter coats the surfaces of minerals that can bond tightly to phosphorus. Once these surfaces are covered, available forms of phosphorus are less likely to react with them. In addition, humic substances may chelate aluminum and iron, both of which can react with phosphorus in the soil solution. When they are held as chelates, these metals are unable to form an insoluble mineral with phosphorus. Plant Nutrition
The Tree of Life, or Etz haChayim (עץ החיים) has upvoted you with divine emanations of G-ds creation itself ex nihilo. We reveal Light by transforming our Desire to Receive for Ourselves to a Desire to Receive for Others. I am part of the Curators Guild (Sephiroth), through which Ein Sof (The Infinite) reveals Itself!
Your post has been voted randomly by @autovoters :)