Metal bucks the trend
The virtually universal relationship in metals between heat and electrical conductivity breaks down spectacularly in vanadium dioxide
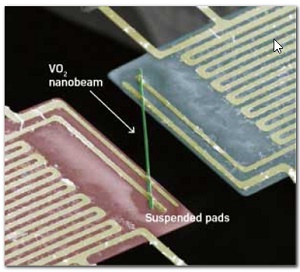
By measuring heat flow from a heating pad (red) to a sensing pad (blue) along a nanorod (green) made of metallic vanadium dioxide, researchers found that the almost universal relation between heat conductivity and thermal conductivity breaks down spectacularly.
By measuring heat flow from a heating pad (red) to a sensing pad (blue) along a nanorod (green) made of metallic vanadium dioxide, researchers found that the almost universal relation between heat conductivity and thermal conductivity breaks down spectacularly.
Researcher has, together with an international team, uncovered an exception to the longstanding rule that effective heat-conducting metals are also good conduits for electricity1. This anomaly could eventually be harnessed in thermoelectric devices that convert waste heat from appliances and engines into useful electric power.
Metals conduct both heat and electricity, which is why they are used to make frying pans as well as electrical wires. This is because electrons can flow freely in metals, carrying thermal energy and electrical charge with them as they move.
This phenomenon is so universal that it has been enshrined in an empirical rule known as the Wiedemann–Franz law, which was discovered more than 150 years ago. While some metals that break this law have been found in recent years, these exceptions usually occur at very low temperatures close to absolute zero, making them impractical for use in applications such as thermoelectric devices and thermal switches.
Now, researcher have found a large deviation from this law at temperatures above room temperature. Specifically, by measuring heat flow along a vanadium dioxide (VO2) nanorod they discovered that the thermal conductivity attributable to the electrons in the material is ten times lower than that predicted by the Wiedemann–Franz law.
The effect was observed in the temperature range −33 to 67 degrees Celsius. Above 67 degrees Celsius, vanadium dioxide suddenly switches from being an electrical insulator to a conductor, accompanied by a small change in its crystal structure.
“This deviation is only possible because of new physics that govern the motion of collective electrons in this unique class of materials, which exhibit a similar metal-to-insulator transition,” says Hippalgaonkar. “In particular, in metallic vanadium dioxide, electrons flow like a liquid, which manifests as them carrying charge effectively, but not heat.” This electron movement contrasts with the more random movement of electrons in a regular metal, which resembles that of molecules in a gas.
It is also possible to tune the heat conduction of vanadium dioxide by introducing tungsten as an impurity. This ability could be exploited in thermal switches.
Excellent write!
Thermal breaks are pivotal in the construction industry, as they inhibit heat transfer across different components of a building, like walls, floors, and windows. These thermal breaks utilize materials with low thermal conductivity to create a barrier that significantly reduces heat flow, thus bolstering the structure's insulation. In features like windows or curtain walls, thermal breaks separate the inner and outer metal frames to curtail direct heat transfer, enhancing energy efficiency. They not only facilitate energy conservation but also reinforce a building's structural integrity, encouraging eco-friendly construction methods, trimming down long-term costs and environmental footprints, and fostering more sustainable and cost-effective buildings.