What are Gene Drives, and How Can They Possibly End Marlaria, Lyme, and Virtually ALL Vector Borne Diseases?
Curing all vector borne diseases is a tall order, but gene drives offer that and so much more.
(Disclaimer: This article is focused on the science behind gene drives and their application. I have tried to condense some material to make it easier to digest and to define some terms for those newer to the field. I left only the material necessary to build up an understanding of why this subject is relevant and how it effects life around us. If there are any questions at all or if you would like more detail, do not hesitate to ask a question in the comments! I would love to discuss the material further.)
DNA connects all life on the planet, save RNA based viruses but we will skip them for today. (Stay tuned for RNA World hypothesis). The majority of your DNA is non-coding, around 90%. The left over codes for all the proteins that allow you to live and grow.
Genes are often grouped as part of the whole, all acting synergistically to survive and propagate through the overall survival of the resulting organism. This may seem to apply to the majority of genes, but it leaves out a crucial class of interesting genetic elements, the selfish ones. The selfish gene theory is a concept in which each gene competes for its own survival, without regard to the organism or species as a whole (Dawkin, 1976). These selfish genetic elements have an interest solely in the replication and propagation of themselves whilst providing no reproductive advantage for its host. That being said, why do they matter and why should we care?
Selfish genetic elements have significant application in gene therapies and as a driver of evolution. Nature has developed significant and powerful mechanisms for repairing and editing DNA. The ability to cut specific sections of DNA and insert desired sequences has been acknowledged for the last half century as invaluable to scientific research. Out of necessity, nature has selected for the creation of cellular machinery to recognize, cut, and repair DNA. Endonucleases represent some of the most important tools for this endeavor, as they recognize and cut DNA at a precise and determined site known as a recognition site (Loenen, et al. 2014).. Originally for the purpose of destroying foreign viral DNA in bacterium, we now use them for a variety of recombination DNA experiments and it has truly revolutionized science.
A specific and separate class of endonuclease known as mega-nucleases or homing endonucleases are selfish. They have unique characteristics that separate them from their common alternatives. Though the function of type two restriction enzymes and homing endonucleases are the same, evidence shows that they may in fact have evolved separately (Belfort and Roberts, 1997). Meganucleases contain DNA recognition sites of 12-40 DNA base pairs, much larger from the 3-8 base pair recognition site of normal restrictive enzymes. As their smaller counter parts do, they cleave DNA to create double stranded breaks. Type two restriction endonucleases are renowned for their extremely specific and precise cuts; this is imparting due to the precision of their binding (Kamps-Hughes, et al. 2013). Their small recognition sequences give no room for error and as a result will only cut at a precise location when bound to that sequence. The large sequences recognized by meganucleases do not share that property (Belfort and Bonocora, 2014). Their large recognition sequences give them high specificity, but with the possibility of recognizing sites with very slight differences in sequence.
Now this is where the fun begins. These endonucleases are encoded on introns. Introns are cut out of RNA products during RNA processing in order to produce a mature mRNA. It is odd for proteins to be coded on introns, but for the megaendoculeases it is essential for its function.
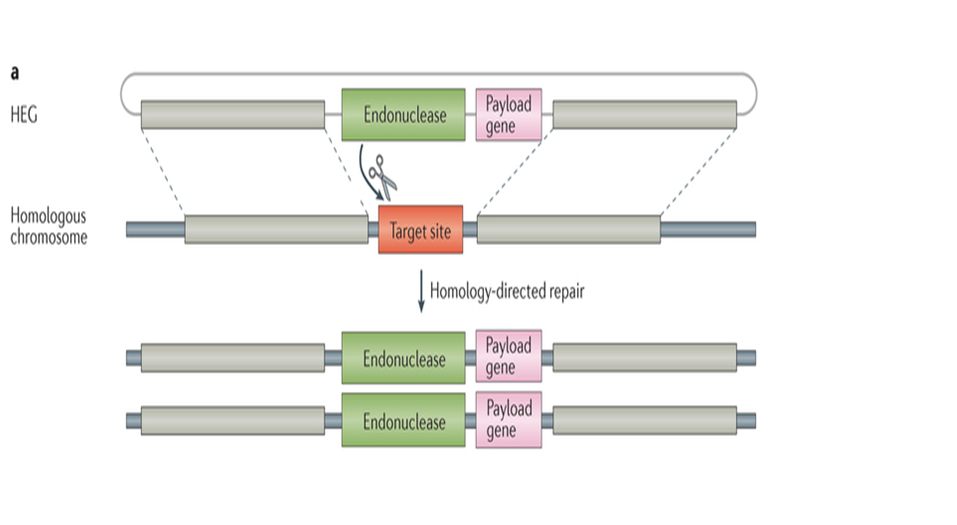
Figure showing different mechanisms of action for megaendonucleases:
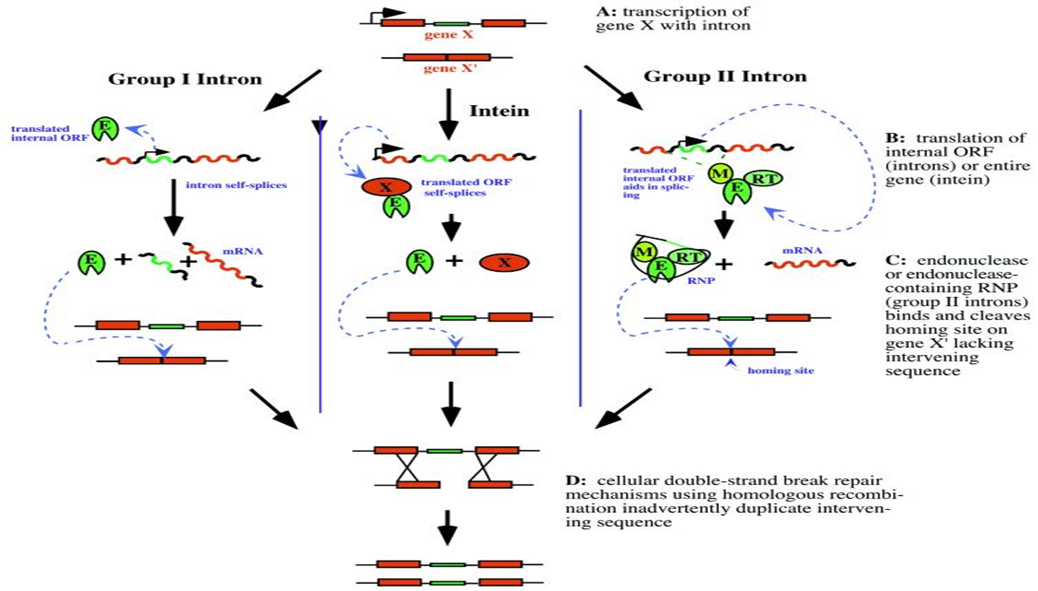
(Chevalier and Stoddard, 2001)
Translation of the open reading frame located on the homing endonuclease intron encodes a meganuclease protein (Chevalier and Stoddard, 2001). Once translated, this is able to recognize regions of the neighboring exons flanking the original intron. Exons are regions of DNA kept during splicing and code proteins usually. These recognition sequences are in general 12-40 bps in length. When it has recognized its homing site, a region of DNA lacking the encoding intron and with the correct sequence matching the flanking exons, the endonuclease creates a double stranded cut. Sensing the damaged DNA, the host cell then recruits cellular machinery to repair the DNA through homologous recombination with the original homing endoculease gene. This creates an additional copy of the gene inserted into a new strand of DNA. This effectively cloned the gene into a new region of DNA that did not have the gene in it before.
THIS IS HUGE!
Since these recognition sites are so large, they will most likely not cut in more than one place on large genomes (Belfort and Bonocora, 2014). With normal endonucleases, they would cut thousands of times on large animal genomes which would result in destroyed DNA. These homing endonucleases allow us to specifically cut DNA at a single location on large organism's DNA. This means that if we can engineer one of these proteins to cut at a desired location, we can make alterations to an animals genome without destroying it.
The possible implications are staggering. By cloning a portion of DNA onto the end of the intron coding for the megaendonuclease, we can insert the desired gene into a specific location in an organisms genome. This selfish gene will insert itself into the target gene, which will knock out its function. This was we can alter harmful genes of organisms and even replace them with working versions. Specifically, pathogen vectors can be targeted (Windbichler, et al. 2011). This means we could target genes of mosquitoes to make them unable to carry Malaria, ticks to carry Lyme, and virtually any other type of vector borne pathogen. This also has implications for those suffering from genetic diseases. We could knock out faulty genes while simultaneously inserting good working versions. This is LIFE CHANGING!
An additional bonus is that these genes do not follow normal laws of Mendelian inheritance (Burt, 2003). Offspring from a parent containing an engineered megaendonuclease will be homologous for that trait. This means that all offspring inherent the trait every time. The reason is that the megaendonuclease seeks out the section of DNA that it is flanked by as a target. If a wild type parent mates with a parent with this endonuclease gene, all of their offspring will be homozygous for the endonuclease gene. It copies onto both sets of chromosomes (Champer, 2016). This is why it is called a gene drive. Even genes that negatively effect an individual will be passed on at a rate of 100% (Windbichler, et al. 2011). These gene drives will spread until all individuals have the gene, and quickly too. With even the introduction of traits with negative effects on a population (Unckless, et al. 2015), the prevalence of a gene drive in a population will be close to 100% from 12-15 generations according to computer simulations and also with laboratory experiments on fruit flies.
Figure of Inheritance due to gene drives:
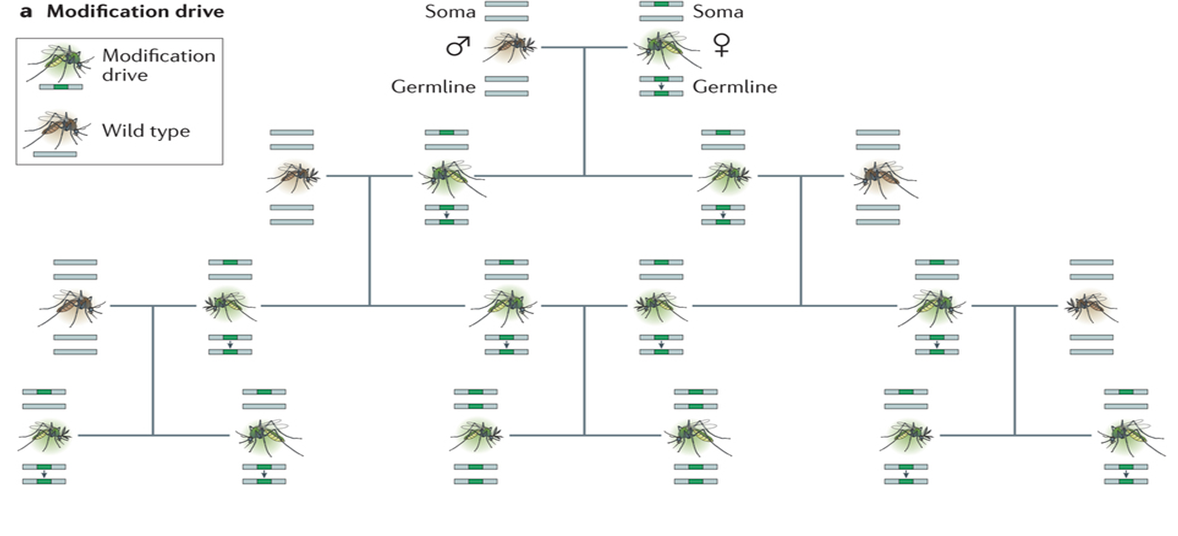
(Champer, 2016)
That is the scary part. Once one of these is released, it is out there. A single missing fly escapes into the wild, and if it mates, all fruit flies around the entire planet will contain the gene drive. Within 12-15 reproductive cycles of a fruit fly... aka under a year. Mess up and release the wrong gene drive lets say for mosquitoes, and you just killed one of the most biologically important insects on the planet. Bats, birds, insects and many other animals prey on mosquitoes. This would have devastating consequences.
Well..... not quite
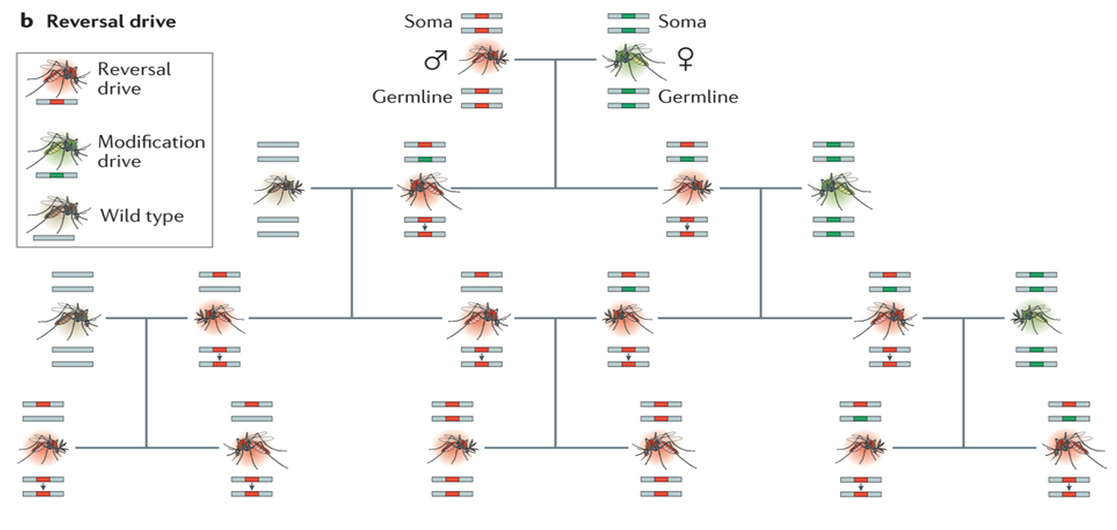
Fortunetly for the mosquitoes, one scientisits major mistake will be a huge oppertunity for another to literally save the planet. A reversal drive is one that inserts itself in the endonuclease gene of the first drive, knocking it out. and replacing it with the new drive (Ledford, 2014). This should in theory correct the original mistake although it has never been proven scientifically (Oye, et al. 2014). This would give scientists an opportunity to save the world.
See that's what scientists are all about, just look at Nye here. (Except in the case of the reversal drive you would be making a substantial and important contribution to the Humanity and actually have a meaningful impact on the planet...)
Luckily 12-15 generations for humans is a long time. We would be able to see if the new trait was effecting people negatively and we could engineer a reversal drive to reverse the effects of the first drive before all humanity is lost.
Figure of a possible reversal drive.
So why are we not using these drives to cure cancer and other human diseases?
This gene drive system lends itself to species that have short sexual reproductive cycles. Organisms like mosquitoes can have 12-15 reproductive cycles in a year, while humans this would be hundreds of years.
The creation of a safe, efficient, and reliable gene therapy has been an uphill battle. For example, The I-SecI homing endonuclease has a recognition sequence of 18 DNA nucleotides (New England Biolabs I-SceI, 2017). For an 18 nucleotide sequence of DNA to be by chance the same as the recognition sequence as I-SecI the probability can be expressed as 1/(4^18). Each position has 4 possible combinations due to the fact DNA is made of 4 nucleotide and at 18 spaces, statistically 1/(4^18) is the appropriate representation of all combinations. However, the property of these endonucleases to cut at regions with slight differences in sequence from its specific recognition site should be cause for concern for large genome organisms. An average human genome being 3 billion bases long (Human, 2010), there is a chance of (1/4^18)/(3^9) or 1/22.9 genomes that the same sequence will appear. That is till pretty bad considering 1 out of 23 people would have an indiscriminate cut by chance in a random section of DNA. What makes this worse is that these proteins have been known to cut with up to 4 mismatched bases (Jurica, et al. 1998). That increases the number of cuts from 1/23 genomes to around 10-100 times per genome. This could be in a vital region of DNA and could cause cancer and other horrible side effects. This might be OK for 1/100 mosquitoes because it doesn't matter if a mosquito develops cancer, but it even one human does.
Diseases associated with the indiscriminate editing of genes can often be severe (Hacein-Bey-Abina, et al. 2003). The accidental insertion of viral vector gene therapy in the LMO-2 gene of a severe combined immunodeficiency patients caused acute lymphocytic leukemia. It was interpreted that the event was caused by insertional mutagenesis. Unintentional insertional mutagenesis mediated by the accidental insertion of a homing endonuclease is a great cause for concern.
Though significant challenges have presented themselves in the endeavor to create effective and safe gene drives and therapies, these genetics systems hold extreme potential. The ability to target large unique sequences of DNA and manipulate cellular machinery to insert foreign DNA into a cell has massive implications. We are holding onto one of the keys to a society free of disease but it will most likely be some time before we are able to use it in a meaningful way.
If you would like a link to any of the source material I used I am happy to provide you with the information you need. Thanks for your time!
For more on scientific breakthroughs and discussions follow me @hutchordie.
Citations and Relevant Information:
Belfort, Marlene, and Richard P. Bonocora (2014). “Homing Endonucleases: From Genetic Anomalies to Programmable Genomic Clippers.” Methods in molecular biology (Clifton, N.J.) 1123 (2014): 1–26. PMC. 2014. From https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4436680/
Belfort, Marlene, and Richard J. Roberts (1997). "Homing endonucleases: keeping the house in order." Nucleic acids research 25.17 (1997): 3379-3388. From https://www.ncbi.nlm.nih.gov/pmc/articles/PMC146926/pdf/253379.pdf
Burt, Austin (2003). “Site-Specific Selfish Genes as Tools for the Control and Genetic Engineering of Natural Populations.” Proceedings of the Royal Society B: Biological Sciences 270.1518 (2003): 921–928. PMC. From https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1691325/pdf/12803906.pdf
Champer, J., Buchman, A., & Akbari, O. S. (2016). Cheating evolution: engineering gene drives to manipulate the fate of wild populations. Nat Rev Genet, 17(3), 146-159. doi: 10.1038/nrg.2015.34 From www.nature.com/nrg/journal/v17/n3/full/nrg.2015.34.html
Chen, Zhilei et al. (2009) “Directed Evolution of Homing Endonuclease I-SceI with Altered Sequence Specificity.” Protein Engineering, Design and Selection 22.4 (2009): 249–256. PMC. From https://www.ncbi.nlm.nih.gov/pubmed/19176595
Chevalier, Brett S., and Barry L. Stoddard (2001). "Homing endonucleases: structural and functional insight into the catalysts of intron/intein mobility." Nucleic acids research 29.18 (2001): 3757-3774. From https://www.ncbi.nlm.nih.gov/pubmed/11557808
Dawkins, Richard (1989). The Selfish Gene. Oxford: Oxford University Press, Print.
Hacein-Bey-Abina, Salima, et al. (2003) "A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency." New England Journal of Medicine 348.3 (2003): 255-256. From http://www.nejm.org/doi/full/10.1056/NEJM200301163480314#t=article
Hafez, Mohamed, and Georg Hausner (2012)."Homing endonucleases: DNA scissors on a mission." Genome 55.8 (2012): 553-569. From http://www.nrcresearchpress.com/doi/pdf/10.1139/g2012-049
"Human Genome Project Completion: Frequently Asked Questions." National Human Genome Research Institute (NHGRI). N.p., 30 Oct. 2010. Web. From https://www.genome.gov/11006943/human-genome-project-completion-frequently-asked-questions/
Jurica, M. S., et al. (1998). B. L. DNA Recognition and Cleavage by the LAGLIDADG Homing Endonuclease I-Cre I. Molecular Cell, 2(4), 469-476. doi: 10.1016/S1097-2765(00)80146-X From https://www.ncbi.nlm.nih.gov/pubmed/9809068
Kamps-Hughes, Nick et al. (2013). “Massively Parallel Characterization of Restriction Endonucleases.” Nucleic Acids Research 41.11 (2013): e119. PMC. From https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3675476/
Ledford, Heidi. "Safety upgrade found for gene-editing technique." Nature News. Nature Publishing Group, 16 Nov. 2015. Web. From http://www.nature.com/news/safety-upgrade-found-for-gene-editing-technique-1.18799
Loenen, Wil A. M., et al. (2014). Highlights of the DNA cutters: a short history of the restriction enzymes. Nucleic Acids Res 2014; 42 (1): 3-19. doi: 10.1093/nar/gkt990 From https://academic.oup.com/nar/article/42/1/3/2438402/Highlights-of-the-DNA-cutters-a-short-history-of
New England Biolabs. "I-SceI." I-SceI | NEB. N.p., n.d. Web. Retrieved 13 Feb. 2017. From https://www.neb.com/products/r0694-i-scei
New England Biolabs. "Restriction Modification Systems." Restriction Modification Systems | NEB. N.p., n.d. Web. https://www.neb.com/sitecore/content/nebsg/home/faqs/cutsmart-restriction-endonucleases/restriction-modification-systems
Nolan, Tony, and Andrea Cristanti (2017). "Using Gene Drives to Limit the Spread of Malaria." The Scientist. Lab X Media Group, 1 Jan. 2017. Web. From http://www.the-scientist.com/?articles.view/articleNo/47755/title/Using-Gene-Drives-to-Limit-the-Spread-of-Malaria/
Oye, Kenneth A., et al. (2014). "Regulating gene drives." Science 345.6197 (2014): 626-628. From http://science.sciencemag.org/content/345/6197/626
Unckless, Robert L., et al. (2015). "Modeling the manipulation of natural populations by the mutagenic chain reaction." Genetics 201.2 (2015): 425-431. From http://www.genetics.org/content/201/2/425
"Vector-borne diseases." World Health Organization. World Health Organization, Feb. 2016. Web. Retrieved . From http://www.who.int/mediacentre/factsheets/fs387/en/
Windbichler, N. et al. (2011). A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature, 473(7346), 212-215. doi: http://www.nature.com/nature/journal/v473/n7346/abs/10.1038-nature09937-unlocked.html#supplementary-information
Yang, Jian, et al. (1996). "Efficient integration of an intron RNA into double-stranded DNA by reverse splicing." Nature 381.6580 (1996): 332. From https://www.ncbi.nlm.nih.gov/pubmed/8692273






A nice and well written, highly technical piece on Gene Drives. Well done, thank you for taking the time to educate the community on this fascinating biotechnology.
As a bonus, and in addition to resteeming for exposure, we are awarding you a small 10 Steem Power deposit as a thank you for creating quality STEM related postings on Steemit. We hope you will continue to educate us all!
I am very grateful and honored for this distinction and for your support! I am pleased that there is a large and welcoming community of scientists and science enthusiasts on this network and I am very happy to contribute to it. I look forward to exploring new the content this community puts forward and I am humbled to be a part of the ever expanding field of science. Thank you!
Fantastic post - hope to read more from you in future! :)
Thanks I am glad you liked it!
I wrote a post quite a while back on the RNA world hypothesis, I am a fan of Carl Woese's work. Though I never did get to meet him.
You could really (and should) make a post discussing endonucleases, their various classes, what make them different, and possibly if you've time or knowledge their respective methylases. How bacteria utilize them (IE explain their purpose as a defense mechanism), and how they have been employed in molecular biology. Mostly as you use the terms, but I think a lot of regular people (and a lot of scientists too!) don't have a particularly good grasp on what these enzymes really are. I mean I could write a piece like that, but there is only so much time in the day, and its nice to see someone else around that might want to contribute to educating others in the same vein that I do.
Another interesting area for discussion would be to talk about transposable elements and transposases. Their is a lot more motility going on in genetic material then people perhaps realize, and more than a few sequences that like to move and shake.
It's a bummer that his show was crappy.
My final suggestion, for a post like this... CITATONS. You really need to provide people with some sort of means to read more (Especially with regards to background) on the things you are talking about. You did a nice job discussing the topics at hand, but I would suspect that most people would be fairly overwhelmed.
Gene drives are an interesting piece of molecular biology "tech" and are interesting for fast replicating things, though I am not so sure I buy your "end virtually all vector borne diseases" pitch.
In all, nice job. :D
Thanks for the feed back, I wasn't sure about citations in this form of presenting material. I am pretty new to steem. I guess it is just a good practice in general. I will most certainly add that in immediately.
Also thanks so much for the material suggestions! I was already thinking I should have done an intro to endonucleases, and maybe even human genetics. I just felt that meganucleases are moderately unknown even to most scientists. i really appreciate the feed back!
If we studied more into vector host interactions and made strains of host animals that are resistant to the colonization of a vector, I don't see it as "too" far fetched to cure most vector borne diseases. Although it is very unlikely the time and effort is going to be spent into doing such research any time soon. You also have to take into account population control as well as a result of curing all illness. VERY tricky area of research.
Yeah, it's also one of my peves with scientific writing on the internet in general. Most authors just do not provide enough bread crumbs for the reader ( and people are lazy, if you don't lay it out for them, they probably won't go find it on their own ). The presence of easily accessible cited material would give people the opportunity to really take much more away from your article.
Talking about more things from a pure genetics standpoint is something that I haven't seen anyone on here do, I wrote some VERY general background pieces on DNA/replication/protein expression/ribosomes in the past. I suppose the way you go about it depends on the audience you are targeting (keep in mind that the audience here is largely non scientists, so boiling down the concepts to be as simple and digestible as possible really aides in people taking positive information away that they could apply to future readings).
Yep, yep, and definitely yep.
I would very much enjoy bringing a geneticist's eye to the party! It is always a challenge providing enough info for those who desire depth and for those who just want a quick break down.
Great topic. Learnt lots that I didn't already know about.
Glad to hear it! I only heard about this topic recently at the very end of my undergraduate education! I researched it on my own, I was never taught it during my education. And I am a Molecular Geneticist no less!!!!!
One of the real advantages of using gene-editing (such as CRISPR-Cas) over viral vectors is that you can precisely determine the insertion site, although there are so excited likely off target effects.
Yes, Crispr-cas is actually a much more novel type of gene targeting. It is very interesting and it too has its side effects and draw backs. I think it is much more plausible that Crispr will be used sooner than megaendonucleases. They are much easier to manipulate and alter. It is really a great subject of research! HUGE opportunity for major scientific advancement in gene therapy field, it is tantalizing!
Great article. Biologically informative 👍
Thanks I am happy you enjoyed it!
It sounds like you are unconsciously advocating for a genetically determinist society.Also, Bill Nye is a propagandist.I suggest you to research more as this article does seem to have an oversimplified view on the topic
In my opinion( which means little) this article does not promote Bill Nye in any way other than to implicitly articulate a point that in popular culture, however brainwashing it may be, is raising these questions and concerns while mainstreaming it on places like netflix. It's a backhanded and snide reference to the desperate scientist, Nye, the unimportant guy.
Not particularly, at least from the perspective of the function of a gene drive.
I specifically was targeting those who may not be well versed in scientific language or have specialized in genetics. I appreciate your concern for material containing appropriate and valuable material. It would take a very long time to explain the intricacies of gene therapy and the problems associated with its implication. This was went as an overview.