Looking for new ways to treat leukemia, genetic therapy may come
Online Desk Standing in front of a new path, medical science The US Food and Drug Administration (FDA) committee has given a new lecture-leukemia- and some other cancer-treatment treatments. This time, just waiting for the approval of the FDA, which seems to be available. After that there will be a multinational company-discovered new medical system-discovered new medical practice. If that is the first gene therapy in the market then it will be the same.
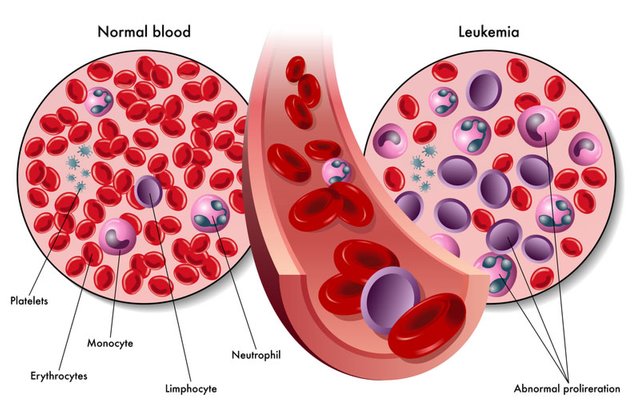
The leukemia-infected patient will be extracted from the most beneficial T-cell. Instead of his genes, the T-cells will be re-invented in the patient's body. This t-cell of gene-replacement T-cell will lose the cancer during this time. The cause of the disease is the disease, so the name given to this method is 'living medicine'.
World-class multinational pharmaceutical companies were so scorched that one ran. To bring such gene therapy. Only one organization, whose funding was run in the laboratory of Pennsylvania University, ran
In the new method, treatment of leukemia and some other cancer, the scientists of that multinational company had trials between April 2015 and 2016, among 63 patients. Of these, 52 patients (8.5%) have recovered, 11 people died. Researchers are very enthusiastic looking at those who have been cured.
Side effects of new treatment? It was a matter of concern for the FDA experts. Because, in the two treatments of cancer - chemotherapy and radioactivity - the anxiety of side effects worries the researchers. On behalf of the multinational company, the FDA panel has been advised that the side effects of treatment in the new procedure are quite low.
The hearing in front of the FDA panel is presented in the new procedure, and many patients who have received a new procedure One of those 12-year-old Emily Whitehead In 2012, when Emily was 6 years old, Emily was admitted to hospital with severe fever, very little blood pressure and chest pain. Emily is now completely healthy after treatment with a new procedure. Emilie's father told the panel, "If the FDA approves this treatment, it will benefit thousands of children. Those who are sitting in the panel, they are going to be witnesses of a historical step. Blood cancer will be treated as a disease. "
The hearing was attended and Dr. McMahon, a leukemia-infected patient's father. The 12-year-old boy, who is suffering from leukemia since his age of 3. Do not treat him in a new procedure because he was not treated with conventional treatment. Someone playing hockey now
And another patient's mother, Amy Kapen, said that her 5-year-old girl Sofia died after being treated in a new procedure. He is not against the treatment of new methods. Because, he is convinced that the girl who saved the sophia treatment at the right time
Spend? Yes, that's enough. At the moment, the cost of the new treatment is three million dollars. Whether it is Yet the new treatment that is hoping to cure disease is a big step in science. The spokesman of the multinational company said, not only in the US, new treatments in many countries
They are waiting for spreading the day.