New Drug Application (FDA) for Only Known Synthetic Aphrodisiac

model23.com has initiated a long position in Palatin Technologies, Inc. (PTN), for an intended duration of at least one year
Bremelanotide (tentative brand name Rekynda; former developmental code name PT-141) is a peptide melanocortin receptor agonist which is under development by Palatin Technologies as a treatment for female sexual dysfunction.
In January 2017, Palatin Technologies successfully completed two phase III clinical trials of bremelanotide for female sexual dysfunction.[2] The developers of the drug have announced their intention to submit a New Drug Application of bremelanotide for female sexual dysfunction in early 2018.
https://en.wikipedia.org/wiki/Bremelanotide
Unlike Viagra and other related medications, it does not act upon the vascular system, but directly increases sexual desire via the nervous system.
https://www.peptidesciences.com/pt-141
Bremelanotide is marketed to treat Female Sexual Dysfunction, but is also effective in men.
Female sexual dysfunction, or FSD, is a multifactorial condition that has anatomical, physiological, medical, psychological and social components. It is defined as persistent or recurring problems during one of more the stages of sexual response, which cause distress or problems to the women.
FSD is a spectrum of disorders that affect women of all ages and across all ethnicities and social groups. It is related to problems of sexual desire, arousal, orgasm and pain. Women may have a single problem or they may have a combination of several different issues. FSD has a significant impact on a patient’s self-image, relationship and/or general well-being. To establish a diagnosis of FSD, these problems must be associated with personal distress, as determined by the affected woman.
Bremelanotide is being developed for a type of FSD with decreased sexual desire that is known as hypoactive sexual desire disorder, or HSDD.
http://www.palatin.com/research-focus/female-sexual-dysfunction/
Palatin exclusively licensed North American rights to develop and commercialize bremelanotide to AMAG Pharmaceuticals, Inc. (Nasdaq:AMAG). The anticipated filing date in the U.S. for a new drug application for bremelanotide, an on-demand treatment for hypoactive sexual desire disorder (HSDD) in premenopausal women, is early 2018, with an anticipated approval and launch by early 2019.
Under the terms of the license agreement, AMAG paid Palatin $60 million of total upfront consideration, and will pay up to $80 million contingent upon achieving certain regulatory milestones and up to $300 million contingent upon meeting certain sales milestones. The first sales milestone is $25 million and would be triggered when bremelanotide annual net sales in North America exceed $250 million. Additionally, AMAG will reimburse Palatin for up to $25 million of ongoing development expenses incurred by Palatin in connection with the remaining development of bremelanotide in 2017. AMAG will also pay Palatin tiered royalties on net sales ranging from high single-digit to low double-digit percentages.
Palatin retains rights to bremelanotide outside North America and is actively seeking licensees.
http://www.palatin.com/research-focus/female-sexual-dysfunction/bremelanotide-license/
Regarding development of bremelanotide outside of the North American market, we will do this only in the context of a partnership. To this end, we are focusing our business development efforts on a bremelanotide partnership with the European Union as well as other regions of the world. And we currently have ongoing discussions with multiple potential partners.
https://finance.yahoo.com/news/edited-transcript-ptn-earnings-conference-211558851.html
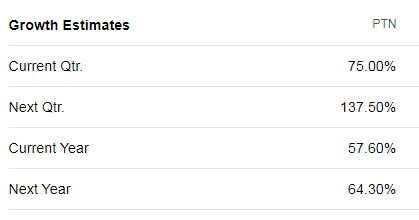
https://finance.yahoo.com/quote/PTN/analysts?p=PTN
Canaccord Genuity analyst John Newman reiterated a Buy rating on Palatin Technologies (AMEX: PTN) on May 16 and set a price target of $6. The company’s shares closed today at $0.43
http://www.analystratings.com/articles/palatin-technologies-gets-a-buy-rating-from-canaccord-genuity/
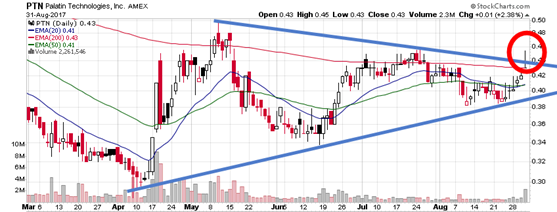
http://stockcharts.com/h-sc/ui?s=PTN&p=D&yr=0&mn=6&dy=0&id=p14658308312
The next PTN earnings date is September 19th.
http://www.nasdaq.com/earnings/report/ptn
Other references:
https://drugs-forum.com/threads/pt-141-the-first-real-honest-to-god-horny-making-body-shaking-aphrodisiac.34647/
Congratulations @model23.com! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP