Fundamentals of Chemistry || Lecture 08 || What are Isotopes?
Isotope is a word that gets thrown around in a chemistry a lot. Isotopes are different versions of an element or different versions of certain kinds of an atom. This can be a tricky concept though and a lot of people get confused by isotopes so, I want to describe them in a simple way. From my previous lectures on Steemit, you would have definitely learned about atomic structure. An atom is made up of three tiny particles like proton, neutron and electron. Protons and neutrons are found in the nucleus while electrons revolve around the nucleus in fixed paths or shells just like planets revolve around the Sun. Remember that electron is a negatively charged particle and proton is positively charged particle and atom as a whole is electrically neutral because the number of protons is equal to the number of electrons which are revolving in shells. The number of protons in any atom refers to its atomic number and number of protons plus number of neutrons in an atom is called its mass number. Now what are isotopes?
Isotopes are the atoms of the same element having same atomic number but different mass number.
As their mass number is different so, they will have different number of neutrons in them. Let me explain this with the examples of isotopes of hydrogen and carbon atom.
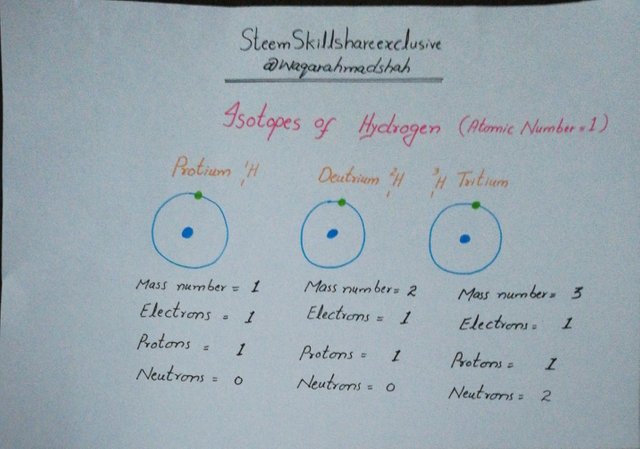
Hydrogen has following three isotopes:
- Protium
- Deutrium
- Tritium
Following pic indicates that how they differ in terms of number of neutrons and mass number.
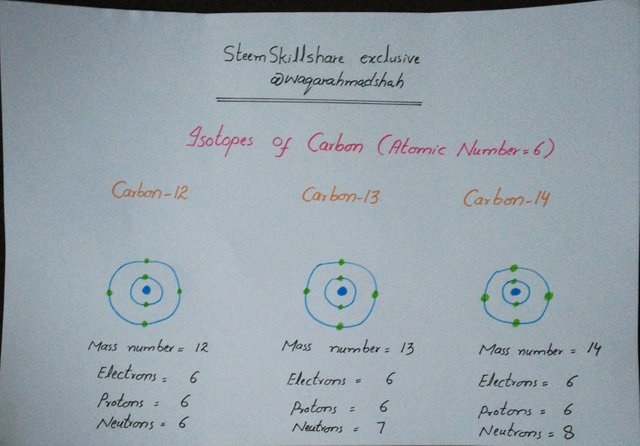
Carbon has following three isotopes.
C-12 or Carbon-12
C-13 or Carbon-13
C-14 or Carbon-14
Following pic indicates that how they differ in terms of number of neutrons and mass number.

Hello my friend. I loved your post. Very timely about the isotopes. I love chemistry, it is so interesting. Cheers!
Hello @waqarahmadshah , your post has been supported by @zmoreno using @steemcurator07 account.
Thank you for making a post in the #Science/ #Computing/ #Technology category. We appreciate the work you have put into this post.
We have analyzed your post and come up with the following conclusion: