Fundamentals of Chemistry || Lecture 05 || Structure of an Atom
Atoms are the fundamental building blocks of all matter. Consider the following: water, sodium chloride, and oxygen. Water is a liquid, sodium chloride is a solid, and oxygen is a gas, as you may know.
.jpg)
.jpg)
.jpg)
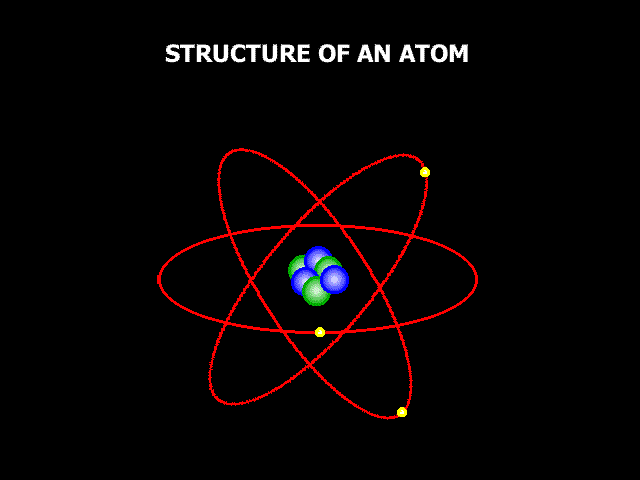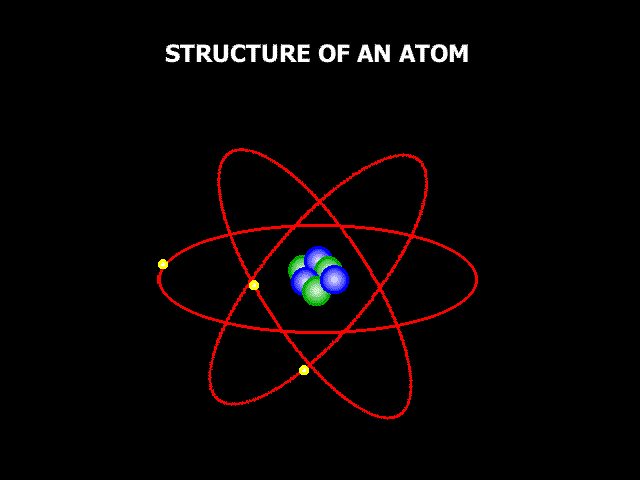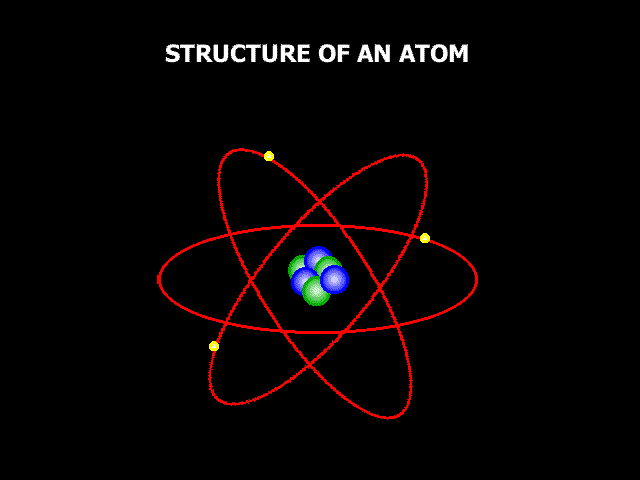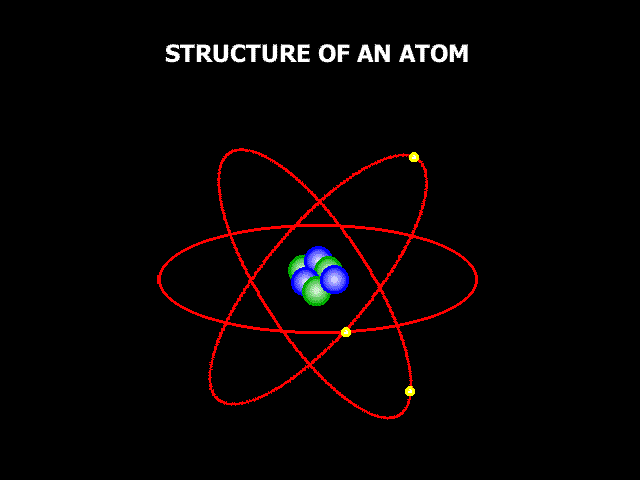
So, if we could zoom in zillions and zillions of times and look at these three things carefully enough, we would be able to see the atoms, these little building components that make everything up. Now, atoms aren't exactly like small circles, but it's a decent way to think about them, and it's how we normally depict them when we're learning about them. Atoms are extremely little; in fact, they are so small that it might be difficult to comprehend how small they are. If you drew a dot on a white board with a board marker, it would contain millions of atoms. The image below shows a magnified picture of an atom.
It's not a perfect illustration of what an atom might look like, but it'll suffice for now. The atom is made up of smaller particles. So, we stated that atoms are the building pieces for everything, but when you look at an atom, you can see that it is made up of even smaller building blocks. So, at the core of the atom lies something called the "Nucleus," and the nucleus is composed of two tiny particles known as "Protons" and "Neutrons." Then there are "Electrons" outside of an atom. Electrons are coupled to these oval routes, as shown in the above image. These oval channels are known as "Orbits" or "Shells," and electrons are continually passing through them at incredible speeds. For a variety of reasons, electric charge is critical when discussing atoms. Protons are positively charged. One proton has a charge of 1+. Electrons, on the other hand, are positively charged. One electron has a charge of one. Neutrons now have no electric charge at all. They have a charge of zero, and neutral is a term used to describe anything that does not have a charge. So neutrons sound similar to neutrals. Positive and negative charges are now attracted to one another. Remember that opposing charges attract, which has crucial implications for how atoms operate. We stated that electrons are travelling around the exterior of an atom at high speeds. So why don't they just go off into space? The reason for this is that negatively charged electrons are attracted to positively charged protons. So, the protons kind of pull the electrons in, keeping them from flying away, but the electrons are going so quickly that they are unable to draw in and touch the protons. That's why they stay outside instead of crashing into the nucleus. The issue now is, why are neutrons essential if they have no charge at all? Neutrons, it appears, help maintain all of the particles in the nucleus tightly bound to one another. So, they are important as well.
That's all from me for today's lecture. I tried my best to give you an understanding about the structure of an atom and its components. I have already made an introductory post as chemistry teacher in this community. The link to that post is given below.
I am looking forward for getting the label of Chemistry Teacher in this community.

Regards:
@waqarahmadshah
Special Mentions

#chemistryteacher #steemskillshare #chemistryonsteemit #steemexclusive #pakistan #club5050 #structureofatom
Thanks for producing this quality post in the community. Please join our discord channel Discord and join our today's meeting at exactly 21: 30 pm UTC prompt.
@abu78 Thank you for appreciating my content. I have been writing posts about chemistry subject in this community but i have not been entitled with Chemistry Teacher label yet so far even though I have made an introduction post about it also. I have already joined the trail and I will also delegate SP once I got enough. I have joined the discord server you mentioned but I was not able to attend the meeting because I was offline.
great, thanks for your time and support in the community and also for drawing my attention to the labeling.
Hi @abu78 Thank you so much for giving me the Chemistry Teacher label. I am feeling motivated now and will continue producing quality posts in this community. One thing i would like to ask about SP delegation since I am still new on this platform. What is the minimum amount for SP delegation in this community? How will I get the label of SP delegation after delegating SP?