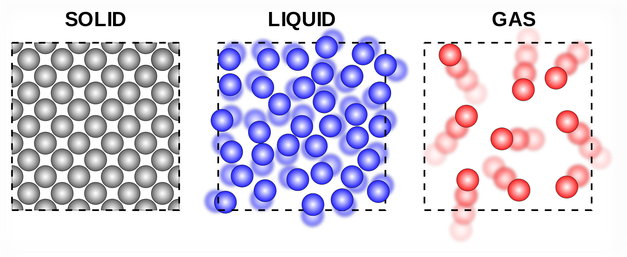
The kinetic molecular theory of matter.
Source
The kinetic molecular theory of matter assumes that matter is made up of atoms that summed up in a molecule.
Before I continue let's refresh her memory with what an atom and the molecule is.
Any smallest particle of an element that can exist alone and yet possesses the chemical properties of that element is known as an atom.
While a molecule is a group of atoms of the same or different elements join together in a simple proportion.
Now to start with the size of the molecule. The size of the molecule is of the order of 0.01m.
The molecule of solid are fixed in definite proportions and they vibrate about a mean or fixed position.

Source
The togetherness of the molecules of solid is because of the intermolecular forces of Attraction and repulsion. so solid has a fixed shape and volume.
The molecule of liquids are more free to move about within the liquids than the molecule of a solid. Although both molecule are held together by intermolecular forces.
In gas, the molecules move randomly due to weak intermolecular Force of attraction. Gas hence retained the shape and volume of its container.
The molecule of a gas are also much further apart than those of solid and liquids.
HERE ARE THE ASSUMPTIONS CONCERNING THE KINETIC MOLECULAR THEORY.
1• All substances are composed of small particles called molecules.
2• That molecules are always moving randomly colliding elastically with one another and changing their direction in states.
3• There is always a force known as force of Attraction between the molecules.
4• The volume of the gas molecules is negligible compared with the volume of the gas container.
5• Gas molecules are perfectly elastic during collisions. When two molecules collide their individual energies may change and one move faster while the other slows down but their total kinetic energy is remain the same that is not kinetic energy is converted to heat.
6• The gas molecules move randomly in straight lines colliding with one another and with the walls of the container.

Nice one
Thank you