Observing pH changes in the electrolysis of a NaCl solution
Electrolysis is a process by which a chemical reaction occurs when electric current is applied to a solution, causing oxidation-reduction reactions at both electrodes. In other words, the opposite of what we can observe in a galvanic cell, in which electric current is produced from a spontaneous reaction, occurs. For example, we know that under certain conditions hydrogen and oxygen combine to produce water; however, if we pass an electric current through the water by means of two electrodes, we will cause its decomposition into hydrogen and oxygen.
Now, during the process changes in pH occur in the proximity of the electrodes that are imperceptible to the eye, but using a convenient pH indicator they can be made evident by interesting color changes around both electrodes in the same solution.
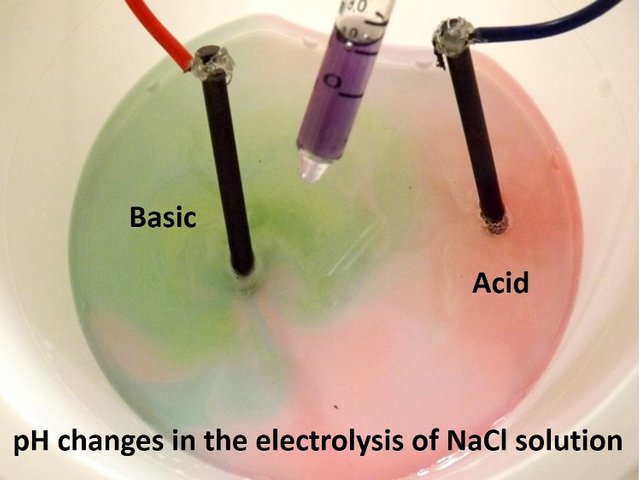
Source: @emiliomoron.
In this post I want to share with you a simple experiment so that we can check this phenomenon, and that we can easily do at home. We will only need:
- A sodium chloride solution, which we obtain by dissolving table salt in water.
- A pH indicator. For this experiment I am using as pH indicator the purple cabbage extract, this contains natural pigments that give it that purple color, but in basic medium they change to green color and in acid medium they become red.
- Two graphite rods, they can be obtained from a pair of pencils or from a pair of used batteries.
- A 9V battery.
- A pair of wires.
- An eyedropper.
In the following video we can check the pH changes that occur in a sodium chloride (NaCl) solution during electrolysis.
The following image describes what happens at the cathode during the electrolysis of the aqueous NaCl solution, which is dissociated into Na+ and Cl- in the solution.
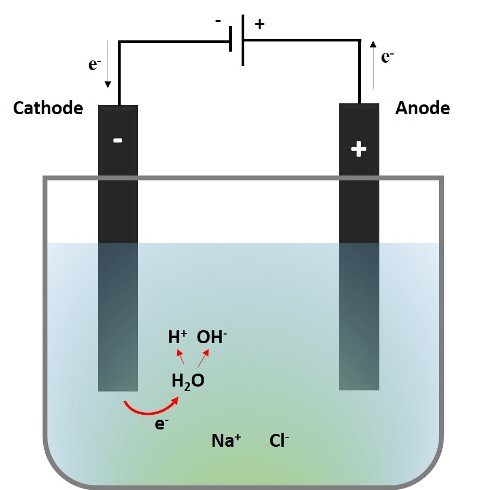
Source: @emiliomoron
As the circuit is established the sodium ions (Na+) will be mobilized to the cathode, but they do not undergo any reaction since the reduction potential of the hydrogen reduction reaction of water is higher, so the reaction that preferably takes place is as follows:
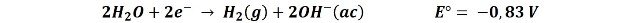
What we observe in the video is that an electron is transferred from the cathode to a water molecule releasing an atomic hydrogen, and with the hydrogen released from another water molecule, they unite to form a hydrogen gas molecule (the bubbles we observe) and the hydroxyl ions (OH-) that give the basic character to the solution, so that in the area near the cathode the pH increases, and the indicator turns from purple to green.
At the anode, however, the process can be a bit more complex, as there are two competing reactions.
1- Oxidation of the oxygen in water to form gaseous oxygen:
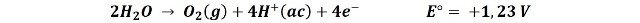
2- The oxidation of the chloride ion to form chlorine gas:
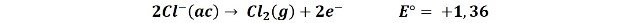
The very close value of the potentials of both reactions make it difficult to predict which of the two reactions will occur, but we can assume that dilute solutions will give rise to the first reaction, releasing oxygen and in concentrated solutions the tendency is to produce chlorine.
So, considering that we are working with a dilute solution of NaCl; the following image represents the process occurring at the anode.
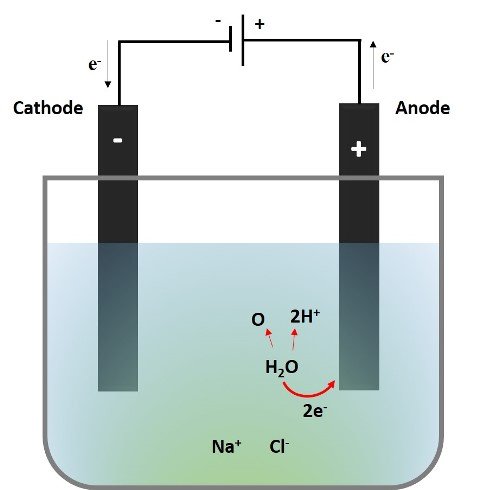
What we see is that two electrons from the oxygen in the water are transferred to the anode, the monoatomic oxygen will combine with another monoatomic oxygen and be released as a gas, while the hydronium ions (H+) released give the acidic character to the solution near the anode, thus changing the color of the indicator from purple to red.
Well friends, this type of material allows us to visualize clearly the processes of oxidation-reduction that take place during the electrolysis of a NaCl solution, and can be used by teachers and students as reference material and educational support, or as a guide to carry out the reaction at home or in the classroom, as it requires very few materials and all are very easy to achieve, in addition, that very visible results are achieved that help to fix the knowledge taught in the classroom.
References
Wikipedia.com. Electrolysis.
Wikipedia.com. Table of reduction potentials.