Formation of iron precipitates. Qualitative analysis of cations
I take this opportunity to share my first contribution in this community that supports science in the social platform Steem, I hope you like the information I bring you, related to the fascinating world of chemistry.
I personally think that the formation of colorful precipitates from a solution is one of the physical changes that most attract the attention of the chemistry student. The sudden appearance of a solid of a different color can amaze many, making this experience a special way to introduce the theoretical content of solubility and precipitates.
We must remember that in chemistry, precipitation is the appearance of a solid phase within a liquid, due to two possible reasons: the addition of a substance capable of reacting with one of the ions present in the solution, giving rise to the formation of an insoluble compound; or, by the increase in the concentration of the same solution until saturation is exceeded. The precipitate is the solid product formed.
Precipitates of iron hydroxide. Source: @emiliomoron.
These precipitates not only serve to amaze us by their sudden appearance, but are the basis of many qualitative analysis procedures of substances, especially for the determination of cations and anions in unknown samples, as well as to achieve their separation from the medium in which they are dissolved, thanks to the fact that there are many substances whose precipitates have a characteristic color.
Solubility
The formation of precipitates involves chemical equilibria between a solid and a liquid phase, and to understand how they proceed we must first understand the concept of solubility.
Surely we have heard that water is the universal solvent, certainly water has the ability to dissolve many substances, but this property is not infinite. A test we can do at home is to dissolve a spoonful of salt in water, as we know this will dissolve, but if we continue adding salt to the same amount of water (keeping the temperature constant) it will reach a point where the liquid will no longer admit more dissolved salt (it will be saturated), and any excess salt will precipitate to the bottom of the glass.
So, when you have a saturated solution at equilibrium, at constant temperature, the maximum amount of solid that can be dissolved by the liquid is the solubility of the liquid, which we can express in g/mL.
Precipitation
If we have an ionic compound, of type AB, dissolved in water and in heterogeneous equilibrium between the solid and the liquid; we will have in turn that the compound will be in equilibrium with its dissolved ions, a condition that we represent according to the following expression:
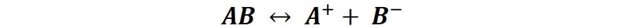
The equilibrium constant for dissociation would be given by the expression:
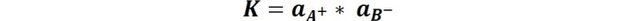
Which is known as the solubility product (Kps). And because the activity of a solid at equilibrium remains constant and the concentrations of a poorly soluble salt are small, the constant is approximated by a:
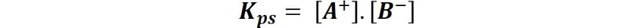
And this expression governs the solubility equilibrium in a solution, so that if in a saturated solution the equilibrium is broken by a decrease in the concentration of its ions, more solid will be dissolved to recover the equilibrium. And if, on the contrary, this equilibrium is broken by the increase of these concentrations, part of the dissolved solid will precipitate to recover the equilibrium; as expressed by Le'Chatelier's principle.
Iron precipitates
To exemplify the concept let us take two solids to work with, iron sulfate, Fe(SO)4, and ferric trichloride, FeCl3. These solids when dissolved in water will contribute iron ions Fe+2 and Fe+3 respectively. And we will precipitate them with sodium hydroxide to observe their color formation.
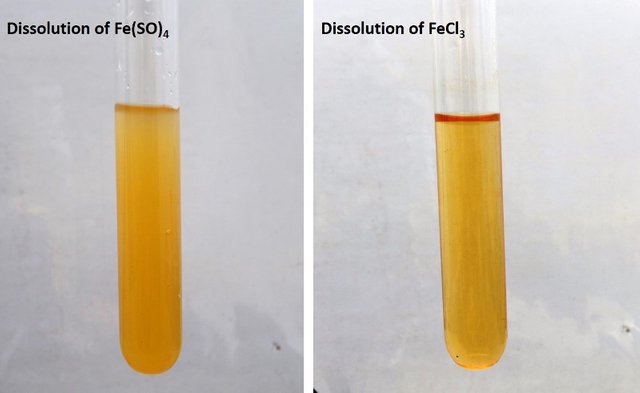
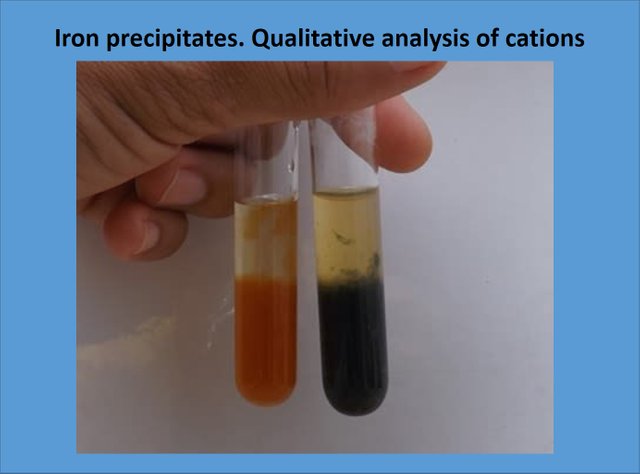
The problem samples are almost identical, how to identify them? Source: @emiliomoron.
Fe+2 precipitate
When adding the sodium hydroxide we can identify which is the cation present in the solution. In the following images we can observe how from the yellow solution of one of the test tubes a dark green precipitate appears after the progressive addition of sodium hydroxide.

Precipitation of ferrous hydroxide. Source: @emiliomoron.
The global reaction between both chemical species can be written as:
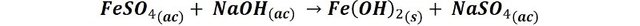
The green solid product formed is iron (II) hydroxide, whose Kps is set at 1,6x10-14. In this case we have prepared solutions whose ion concentrations exceed this value, therefore the solid product precipitated.
Fe+3 precipitate
In these images we can see how from the yellow colored ferric trichloride solution a reddish precipitate is formed after the addition of sodium hydroxide.

Formation of the ferric hydroxide precipitate. Source: @emiliomoron.
The overall reaction is written as follows:
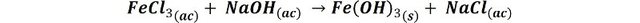
In this case iron(III) hydroxide is formed; similar to the previous example the product of the ion concentrations exceeds the Kps, so the solid precipitates to counteract this change and re-establish equilibrium.
As we see it is easy to identify by the color of the solid formed the identity of the compound, being notably recognizable both iron hydroxides by their characteristic colors. What comprises part of a characterization task, now the separation of the solid compound can occur by filtration, and thus obtain the solid phase to complete gravimetric type analysis and quantify the amount of ions present in a solution.
Well friends, I hope you liked my post. See you next time!
References
Skoog, D.; West,D; Holler, F.J; Crouch, S. (2001). Química analítica. McGraw-Hill Interamerica editores
Chang, R. (2010). química. Decima edicion . McGraw-hill Interamericana editores.
Atkins, P.; Jones, L. (2005). Principios de química. Tercera edición. Editorial medica panamericana