ANTIOXIDANT ACTIVITIES OF PLANTS; ONE OF THE BENEFITS OF NATURE
Oxidative stress is caused by accumulation of free radicals in human body. The presence of free radicals within the body causes heart disease, congestive heart failure, cerebrovascular accidents, and diabetic complications. Many synthetic antioxidants such as butylated hydroxyl toluene, butylated hydroxyl anisol and tetrabutyl hydroquinone are commonly used in scavenging the radicals. However, these synthetic antioxidants are expensive, not readily accessible and are associated with side effects such as; tumor and nausea. Plants are known to be rich in antioxidant. Free radical-mediated diseases are on the increase worldwide and this has brought about the urgent search for novel, cheap and readily available antioxidants from natural sources. Some essential oils from leaves of Withania somnifera, Eclipta prostrate L. and Myrtus communis L. have demonstrated similar antioxidant property against free radicals. The activity of the oils are attributable to the presence of phenolic compounds such as; linalool, methyl eugenol and 1,8 cineole in the oil.
A free radical is any chemical species possessing one or more unpaired electrons, an unpaired electron being one that is alone in an orbital. The simplest radical is the hydrogen atom. Electrons are more stable when paired together in orbital: the two electrons in a pair have different directions of spin. Hence, radicals are generally less stable than non-radicals, although their reactivity varies. Free radicals are capable of reacting indiscriminately with any molecules with which they come in contact. Once radicals are formed they can either react with another radical or with another non radical molecule by various interactions. If two radicals collide they can combine their unpaired electron, thus forming a covalent bond. However, most molecules found in vivo are non-radicals. In this case a radical might donate its unpaired electron to the other molecule, or might take one electron from it, thus transforming its radical character.
Oxygen is essential to aerobic life but, paradoxically, it can be toxic even at atmospheric concentrations. Reactive oxygen species (ROS) and Reactive nitrogen species
(RNS) are formed as byproducts of normal metabolism in aerobic organisms. ROS is a broader term; it includes many reactive species, e.g., superoxide (O2•), hydroxyl (OH•), peroxyl (ROO•), alkyl radical, alkoxyl (RO•) radicals, singlet oxygen (O) and semiquinone radical (HQ•), and ozone(O3). Hydroxyl radicals are formed in the presence of metals and hydrogen peroxide; peroxynitrite might play a small role in hydroxyl radical formation. In this process, certain non-radicals are also produced that are either oxidizing agents or easily converted into radicals, such as HOCl, ozone, H2O2, and lipid peroxides with no unpaired electrons. H2O2 and lipid peroxides also serve as a source of highly reactive •OH, ROO•, and RO• radicals. O2•− reacts quickly with very few molecules, whereas hydroxyl radical OH• has an extremely high rate of reactivity. Reactive nitrogen species include: Nitric Oxide (NO.), Nitrogen dioxide (NO2. radicals and certain non-radicals like: Peroxynitrite (ONOO-), Alkyl peroxynitrites (ROONO), Dinitrogen trioxide (N2O3), Dinitrogen tetroxide (N2O4), Nitrous acid (HNO2). It has been established that ROS can be both harmful and beneficial in biological systems depending on the environment and concentration. Beneficial effects of ROS involve the physiological roles in cellular responses to noxia such as defense against infectious agents, and in the function of a number of cellular signaling systems and gene expression.
NOTE: In contrast, at high concentrations, ROS can mediate damage to cell structures, including lipids and membranes, proteins and nucleic acids; this damage is often referred as “oxidative stress”.
The harmful effects of ROS are balanced by the action of antioxidants, some of which are enzymes present in the body. Despite the presence of the cell’s antioxidant defense system to counteract oxidative damage from ROS, oxidative damage accumulates during the life cycle and has been implicated in diseases, aging and age dependent diseases such as cardiovascular disease, cancer, neurodegenerative disorders and other chronic conditions.
Normal metabolic processes in all the aerobic conditions constitute a major source of ROS. The cellular sources include the electron transport chain of mitochondria and endoplasmic reticulum. ROS are produced by all cell types, e.g., the neutrophils, monocytes, macrophages, and the cytotoxic lymphocytes, and can be formed by the action of many enzymes. The important enzymatic sources responsible for ROS production include NAD(P)H oxidase, xanthine oxidase (XO), and uncoupled form of nitric oxide synthase (NOS).
WHAT ARE ESSENTIAL OILS?
Essential oils are concentrated volatile aromatic compounds produced by plants, the easily evaporated essences that give plants their wonderful scents. Each of these complex precious liquids is extracted from a particular species of plant life. These oils are frequently referred to as the “life force” of plants. Unlike fatty oils, these "essential" oils are volatile, highly concentrated, substances extracted from flowers, leaves, stems, roots, seeds, bark, resin or fruit rinds. The amount of essential oils found in these plants can be anywhere from 0.01 percent to 10 percent of the total. That's why tons of plant material are required for just a few hundred pounds of oil. These oils have potent antimicrobial factors and wide range of therapeutic constituents. These oils are often used for their flavor and their therapeutic or odoriferous properties, in a wide selection of products such as foods, medicines, and cosmetics. These are extracted from oil 'sacs' in flowers, leaves, stems, roots, seeds, wood and bark. They differ significantly from the well-known vegetable, nut and seed oils which are made up of various fatty acids (essential oils are not).
ROLES ANTIOXIDANTS
Essential oils are used by the plants in the same way they are by humans , they fight infection, contain hormone-like compounds, initiate cellular regeneration, and work as chemical defense against fungal, viral, and animal foes. Despite their foliar origins however, essential oils have a similar structure to some compounds found in blood and tissues, making them compatible with human physiology.
Extraction of Essential Oils
Essential oils can be extracted from several plants with different parts by various extraction methods. The manufacturing of essential oils, and the method used for essential oil extraction are normally dependent on botanical material used. State and form of material is another factor used for consideration. Extraction method is one of prime factors that determine the quality of essential oil. Inappropriate extraction procedure can lead to the damage or alter action of chemical signature of essential oil. This results in the loss in bioactivity and natural characteristics. For severe case, discoloration, off-odor as well as physical change such as the increased viscosity can occur. These changes in extracted essential oil must be avoided. There are several methods used in the extraction of essential oils from plants, common methods are:
Steam Distillation
Steam distillation is the most widely used method for plant essential oil extraction. The proportion of essential oils extracted by steam distillation is 93% and the remaining 7% can be further extracted by other methods. Basically, the plant sample is placed in boiling water or heated by steam . The heat applied is the main cause of burst and break down of cell structure of plant material. As a consequence, the aromatic compounds or essential oils from plant material are released. The temperature of heating must be enough to break down the plant material and release aromatic compound or essential oil. A new process design and operation for steam distillation of essential oils to increase oil yield and reduce the loss of polar compounds in wastewater was developed by Masango in 2005. The system consists of a packed bed of the plant materials, which sits above the steam source. Only steam passes through it and the boiling water is not mixed with plant material. Thus, the process requires the minimum amount of steam in the process and the amount of water in the distillate is reduced. Also, water soluble compounds are dissolved into the aqueous fraction of the condensate at a lower extent.Hydrodistillation; has become the standard method of essential oil extraction from plant material such as wood or flower, which is often used to isolate non water-soluble natural products with high boiling point. The process involves the complete immersion of plant materials in water, followed by boiling. This method protects the oils extracted to a certain degree since the surrounding water acts as a barrier to prevent it from overheating. The steam and essential oil vapor are condensed to an aqueous fraction. The advantage of this technique is that the required material can be distilled at a temperature below 100 °C.
Solvent Extraction
Conventional solvent extraction has been implemented for fragile or delicate flower materials, which are not tolerant to the heat of steam distillation. Different solvents including acetone, hexane, petroleum ether, methanol, or ethanol can be used for extraction. For general practice, the solvent is mixed with the plant material and then heated to extract the essential oil, followed by filtration. Subsequently, the filtrate is concentrated by solvent evaporation. The concentrate is resin (resinoid), or concrete (a combination of wax, fragrance, and essent ial oil). From the concentrate, it is then mixed with pure alcohol to extract the oil and distilled at low temperatures. Thealcohol absorbs the fragrance and when the alcohol is evaporated, the aromatic absolute oil is remained. However, this method is a relatively time-consuming process, thus making the oils more expensive than other methods. The ethanol, methanol, and water extracts exerted significant free-radical scavenging activity. Moreover, it has been reported that the water extract exhibited higher antioxidant activity than other extracts (hexane, dichloromethane, ethyl acetate, and methanol). However, solvent residue could be retained in the final product due to incomplete removal. This may cause allergies, toxicity, and affect the immune system.Supercritical Carbondioxide
Conventional methods including solvent extraction and steam distillation have some shortcomings such as long preparation time and large amount of organic solvents. Moreover, the losses of some volatile compounds, low extraction efficiency, degradation of unsaturated compounds, and toxic solvent residue in the extract may be encountered. Therefore, supercritical fluids have been considered as an alternative medium for essential oil extraction. Carbon dioxide (CO2) is the most commonly used supercritical fluid because of its modest critical conditions. Under high-pressure condition, CO2turns into liquid, which can be used as a very inert and safe medium to extract the aromatic molecules from raw material. No solvent residue remains in the final finished product since the liquid CO2 simply reverts to a gas and evaporates under normal atmospheric pressure and temperature.Solvent-free Microwave
The disadvantages of conventional methods such as solvent or hydrodiffusion extraction are the losses of some volatile compounds, low extraction efficiency, long extraction time, degradation of unsaturated or ester compounds through thermal or hydrolytic effects, and toxic solvent residue in the extract . These disadvantages have led to the consideration of the use of solvent-free microwave extraction, SFME. It is a rapid extraction of essentia l oils from aromatic herbs, spices, and dry seeds. SFME has several advantages, involving higher yield and selectivity, shorter time, and environmental friendly. SFME is a combination of microwave heating and dry distillation, performed at atmospheric pressure without any solvent or water. Isolation and concentration of volatile compounds are performed by a single stage. When microwave power at 662W was used in SFME, process time was reduced by 80%, compared with conventional process.Expression or Cold Pressing
This is used to extract the essential oils from citrus rinds such as orange, lemon, grapefruit and bergamot. This method involves the simple pressing of the rind at about 120oF to extract the oil. The rinds are separated from the fruit, are ground or chopped and are then pressed. The result is a watery mixture of essential oil and liquid which will separate given time. Little, if any, alteration from the oil's original state occurs – these citrus oils retain their bright, fresh, uplifting aromas like that of smelling a wonderfully ripe fruit. It is important to note that oils extracted using this method have a relatively short shelf life.Enfleurage
This is an intensive and traditional way of extracting oil from flowers. The process involves layering fat over the flower petals. After the fat has absorbed the essential oils, alcohol is used to separate and extract the oils from the fat. The alcohol is then evaporated and the essential oil collected.Cohobation
Cohobation is a procedure that can only be used during water distillation or water and steam distillation. It uses the practice of returning the distillate water to the still after the oil has been separated from it so that it can be re-boiled. The principal behind it is to minimize the losses of oxygenated components, particularly phenols which dissolve to some extent in the distillate water. For most oils, this level of oil loss through solution in water is less than 0.2%, whereas for phenol-rich oils the amount of oil dissolved in the distillate water is 0.2%-0.7%. As this material is being constantly re-vaporized, condensed and re-vaporized again, any dissolved oxygenated constituents will promote hydrolysis and degradation of themselves or other oil constituents. Similarly, if an oxygenated with a direct heat source or side of a still, which is considerably hotter than 100° C, then the chances of degradation are enhanced. As a result, the practice of cohobation is not recommended unless the temperature to which oxygenated constituents in the distillate are exposed is no higher than 100° C.
Analysis of Essential Oils
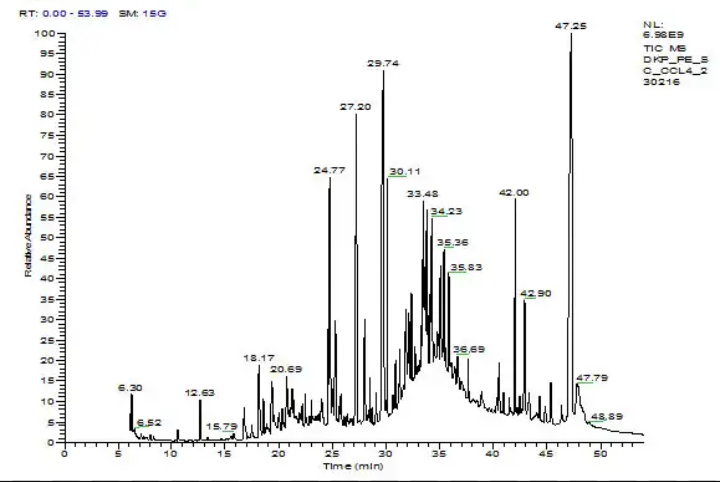
The most common technique employed in the chemical characterisation of essential oils is gas chromatography coupled with mass spectrometry (GC-MS). The relatively small size of essential oil components means that they are all volatile and can therefore be separated according to boiling points. This process takes place in a long thin column (i.e., 30 m) that has the appearance of coiled wire. This column is prepacked with a porous stationary phase that is either polar (slightly charged), such as a wax column (polyethylene glycol; i.e., DB-wax, Carbowax 20M, PEG-20M) or apolar, such as polymethylsiloxane (i.e., HP-1MS or 5% diphenyl- i.e., HP-5MS). The apolar HP-5MS column is the most commonly used. The essential oil is diluted into a solvent and injected into a heated injection chamber ( i.e., 300 °C) so all components of the essential oil are evaporated and delivered by a benign (non-reactive) gas (i.e., nitrogen or helium) to the start of the column, which is usually at a lower temperature (i.e., 60 °C), so essential oil components precipitate onto the column.
Gas Chromatography
Given that essential oils are mixtures of compounds of different molecular weights, from the most volatile hydrocarbons of ten carbon atoms, called monoterpenes, to oxygenated compounds of 15 atoms of carbon, or sesquiterpenes, it is necessary to start with low temperatures that allow the separation of the most volatile ones, then raise it 5 °C or 10 °C per minute to reach the temperature of 200°C to achieve the elution of the heaviest terpenoids. If the chromatography was performed isothermally at low temperature (60°C), the components were not separated from each other, migrating as a single, broad peak and employing long elution times. If, on the other hand, the isothermal run was done at high temperatures (200°C), the peaks were slender and the elution time shorter but proper peak migration and separation was not achieved.
In gas chromatography, separation is performed by heating the column in an oven, most commonly employing a programmed temperature ramp, from a lower to a higher temperature; however, occasionally isothermal (constant temperature) programs are used. When a temperature program is used, the separation of components occurs when the temperature is raised to each of the component’s individual boiling points. At this point the component vaporises and is carried by the non-reactive gas to the detector. The most common detector used in gas chromatography is a mass spectrometer, but flame ionisation detection (FID) is often used where the accuracy of quantification is a concern.Gas Chromatography-Mass Spectrometry
In using FID, the identity of the compound cannot be known. However, if the identity of the compound and its retention time (how long it takes to come out of the column) is already known from a previous experiment, then GC-FID can be used to calculate an accurate relative abundance of each component in the essential oil. Generally prior to GC-FID, GC-MS is used to identify components in the essential oil. Although GC-FID gives more accurate quantification data, it is more common to use the less accurate quantification method, which is calculated from the GC-MS chromatogram.
In a GC-MS chromatogram the retention time of components generally reflects their sizes and the presence of functional groups. The elution of components starts with monoterpenes (10 carbon compounds), which is then followed by oxygenated monoterpenes and finishes with sesquiterpenes (15 carbon compounds). In mass spectrometry the separated component is fragmented by electron impact ionisation, which produces a spectrum of ions that are separated according to mass, with heavier components exerting a greater inertial resistance to a magnetic field than lighter components. In the process of magnetic deflection of their paths, ions are diverted onto a detector. The result is a spectrum showing ions of different sizes with different relative abundances. This spectrum can be generally considered a fingerprint of each component in the essential oil. Because of the reproducibility of this experiment, each mass spectrum can be compared across a spectral library. Using other pieces of information, such as retention time, a fairly reliable match can be made, with minor exceptions. After GC-MS analysis is performed, where discrepancies in identification are involved, essential oil components can be purified using flash chromatography before analysis using nuclear magnetic resonance spectroscope (NMR). Flash chromatography is achieved by packing silica gel (polar phase) into a vertical column with a solvent mixture. The essential oil is added to the top of the column. Because each component has a different binding affinity to the silica gel, the components can be subsequently washed out individually by the same solvent under pressure. The solvent mixture is customised relative to the polarity of the target compound to optimise separation.
Chemical Composition of Essential Oils
Essential Oils are very complex natural mixtures which can contain about 20-60 constituents at quite different concentrations. They are characterized by 2 or 3 major constituents at fairly high concentrations (20-70%) compared to other constituents present in trace amounts. The main constituents of essential oil belong to a class of secondary metabolites called Terpenoids (i.e. monoterpenoids and sesquiterpenoids). The building blocks for the biosynthesis of both monoterpenoids and sesquiterpenoids are isopentenyl pyrophosphate(IPP) and dimethylallyl pyrophosphate (DMAPP).
Bioactivities and Applications of Essential Oils.
An essential oil may contain many hundred chemical elements and this advanced mixture of compounds offers the oil its characteristic fragrance and flavour. The plant components will be extracted with organic solvents to supply oleoresins, concretes and absolutes or extracted with close to or critical solvent like dioxide to supply terribly prime quality extracts. These oleoresins and extract s contain not solely the volatile oil however additionally the targeted non-volatile flavour elements and these have wide application within the food and pharmaceutical industries.
Essential oils as Antioxidant Agent
A free radical is defined as any atom or molecule possessing unpaired electrons. The reactive oxygen species are oxygen derived free radicals such as hydroxyl (OH.), hydroperoxyl (OOH.), peroxyl (ROO.), and alkoxyl (RO.) radicals and non free radicals such as hydrogen peroxide, hypochlorous acid, ozone and singlet oxygen. It can be formed in the living organisms by both endogenously (respiration, peroxisomes stimulation of polymorphonuclear leucocytes and macrophages) and exogenously (ionizing radiation, tobacco smoke, pollutants, pesticides and organic solvents). These free radicals are produced by our body and to stabilize the body’s natural function, but the excess amount could cause cell and tissue damage. It can cause oxidative damage to proteins, lipids, DNA and chronic diseases such as cancer, diabetes, aging and other degenerative diseases in humans.
An antioxidant can be broadly defined as any substance that delays or inhibit oxidative damage to a target molecule. The characteristic feature of an antioxidant is ability to scavenge free radicals due to their hydrogen donators and singlet oxygen quencher. The free radical can be scavenge by natural (plants) and synthetic (butylated hydroxyl toluene, butylated hydroxyl anisol and tetra butyl hydroquinone) antioxidants. But the usages of these synthetic antioxidants are now replaced because the natural antioxidants could be considered as safer without any side effects. The human body has defense mechanisms against free radicals present in most of the cells. Essential oils are rich in phenolic compounds, and for this reason, attract investigators to gauge their activity as antioxidants or free radical scavengers in the DPPH radical assay at room temperature. Kumar et al in their study reported that alcoholic extract of Gossypium herbaceum provided a comprehensive profile of the antioxidant activity; with respect to its phenolic content. G herbaceum reduces the free radical to corresponding hydrazine when it reacts with hydrogen donors in antioxidant principle.Essential oils as Antibacterial Agents
Essential oils will act as antibacterial agents against a wide spectrum of morbific bacterial strains, including: Listeria innocua, Salmonella typhimurium, Escherichia coli, Shigella dysenteria, Bacillus cereus and Staphylococcus aureus etc. Also, essential oil can inhibit some pathogenic bacterial strains, such as Staphylococcus aureus, Escherichia coli, Candida albicans and Helicobacter pylori.Essential oils as Antifungal Agents
It has been estimated that many of the people in industrialized countries suffer from a food borne illness annually. One such chance is the use of essential oils as food additives that may act as antibacterial and antifungal additives. The antimicrobial activity of essential oils was monitored by the macrodilution technique. The mycelia growth inhibition, fungi static and fungicidal concentrations were recorded for each strain that showed sensitivity to the essential oils.
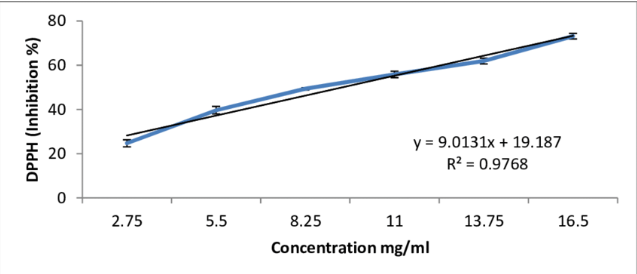
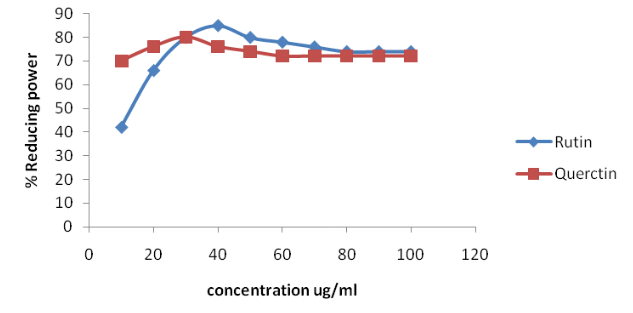
There are several methods used to investigate the antioxidant activity in essential oils. Common methods used are DPPH Assay and Ferric reducing power assay.
DPPH ASSAY
The DPPH radicals are widely used to investigate the scavenging activity of some natural compounds. In the DPPH assay, the antioxidants are able to reduce the stable radical DPPH tothe yellow coloured diphenyl-picrylhydrazine. The method is based on the reduction of alcoholic DPPH solution in the presence of a hydrogen-donating antioxidant due to the formation of the non-radical form DPPH-H by the reaction. The method helps to determine the antiradical power of an antioxidant by measuring of a decrease in the absorbance of DPPH at 517 nm.
FERRIC REDUCING POWER ASSAY
The Ferric reducing power assay uses antioxidants as reductants in a redox-linked colorimetric method employing an easily reduced oxidant, Fe (III). Substances, which have reduction potential, react with potassium ferricyanide (Fe3+) to form potassium ferrocyanide (Fe2+), which then reacts with ferric chloride to form ferric ferrous complex that has an absorption maximum at 700 nm. The absorption readings are related to the reducing power of the electron-donating antioxidants present in the test compound.
References
Oxidative stress and antioxidants
Essential oils and antioxidants
The Chemical Composition of Essential Oils from Cinnamomum camphora and Their Insecticidal Activity against the Stored Product Pests
INTERNATIONAL JOURNAL OF PHARMACEUTICAL SCIENCES AND RESEARCH
Thanks for reading.............
Kindly upvote, comment, follow and resteem





Ilikest....😉
Thanks
Great work there.... nice post
This post selected and upvoted by
Thank you
Congratulations, your post was randomly selected to receive 50+ free upvotes!
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by omotayosteem001 from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.
You got a 12.50% upvote from @ubot courtesy of @omotayosteem001! Send 0.05 Steem or SBD to @ubot for an upvote with link of post in memo.
Every post gets Resteemed (follow us to get your post more exposure)!
98% of earnings paid daily to delegators! Go to www.ubot.ws for details.