Clinical Trials: An Overview
Hi there. This post is my attempt on talking about clinical trials. I have been asked to participate in a few clinical trials in the past but I have declined. My knowledge of clinical trials is based on information I remember, some research and on my time in a statistics course named Survival Analysis For Health Research. This textbook was used for the course.

Clinical Trials
You might have seen a couple of ads or postings which want certain people for various psychology studies. In those studies, the participants had to meet certain criteria and had to perform various tasks. Researchers would gather and analyze the data and see if the results match with what they want. In most cases, the participants are compensated for their time with money, gift cards, etc. Think of clinical trials as something similar to the psychology studies.
Common uses of clinical trials include drug testing and comparing treatments. From two groups of patients, one group of patients would be under the current treatment/drug while the second group would test a new drug/treatment. (It is assumed that the patients have given consent.) The results of the long and research intensive study are examined and decision makers determine whether this new treatment/drug is safe enough for it to be the new standard.
Results from clinical trials can vary. These trials may find that the new treatment/drug improves the patient's health, does nothing or does harm to the patient (unfortunately). With any of these cases, the information gain is important as it can help health professionals improve patient care and make progress in research (i.e. cancer research).
A lot of the participants in clinical trials are volunteers. Willing people are always needed for clinical trials as more data/information can be gathered (larger sample size).

Statistics - Survival Analysis
Statistics does a play a role alongside biology, health sciences, chemistry and medicine when it comes to clinical trials. With clinical trials, we are dealing time to event data where patients may be cured or be exposed to illness at a point in time. The field of statistics that deals with time to event data is called Survival Analysis.
Many of us would prefer to have full information about many things. With clinical trials, you may not know the actual survival time or the time of the event. You may have some people who withdraw from a clinical trial at any time point during the study for various reasons. The event of interest can occur after the end of the study or between follow up times. These are examples of censored information as we do not know the exact survival times.
Kaplan-Meier Curves
A common graphical tool that is used to compare two or more groups is called the Kaplan-Meier Curve. The "curves" start at 1 (100% survival) at the start of the clincial trial and it decreases until the end of the study. The decreases occur as people withdraw from the trial or when the particpant (patient) experiences an event (cure/illness). Times can be in minutes, seconds, days, weeks, months or years.
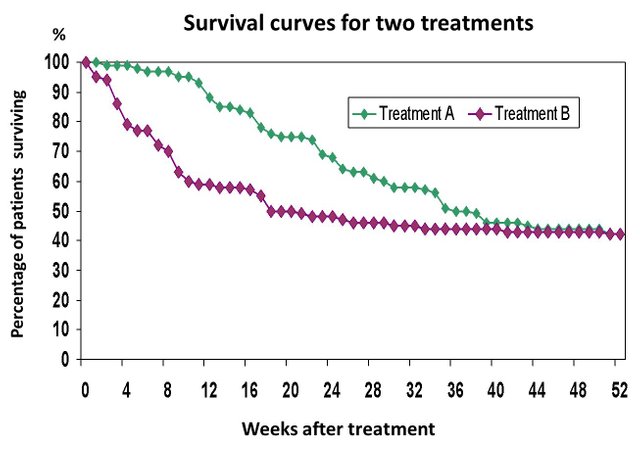
References
- https://www.nhlbi.nih.gov/studies/clinicaltrials/
- http://www.webmd.com/a-to-z-guides/clincial-trial-guide-patients#1
- https://www.livestrong.org/we-can-help/planning-medical-care/considering-clinical-trials
- https://www.nih.gov/health-information/nih-clinical-research-trials-you/basics
- Survival Analysis - A Self-Learning Text by Kleinbaum and Klein
- Understanding Clinical Trials - Youtube Video
Information is not knowledge.
- Albert Einstein
This gem of a post was discovered by the OCD Team!
Reply to this comment if you accept, and are willing to let us promote your gem of a post!
If you accept this, you'll be nominated and the members of the OCD team will vote on whether we'll feature your post in our next compilation post.
You can follow @ocd – learn more about the project and see other Gems! We strive for transparency.
I'm mostly curating in #science and am always glad to see quality posts like yours peeking out between the garbage and plagiarism!
Accepted. Thank you.
You weren't chosen, sorry. But I'll keep an eye out for your posts :)
Thanks for this breakdown!
You are welcome. I am glad you like the information in my post.