Greenhouse Effect - Energy Trapped by the Atmosphere

In a previous post I had mentioned the inability of carbon dioxide and water vapor to trap all the energy radiating from the earth back toward space. While most articles on global warming don't come out and say that all the energy from the earth is trapped by the atmosphere, it is implied in most of them. The truth is, it's just not physically possible.
Remembering your classes in high school science we all learned that atoms are comprised of a nucleus with proton and neutrons and circled by electrons. Energy is absorbed in an atom or molecule when a photon of energy joins with the atom by moving an electron from a lower energy orbit around the atom nuclei to higher energy level orbit. Due to the simplicity of the atoms making up the gases in the atmosphere, there are very limited jumps for low energy orbits to higher energy orbits. To make these jumps, the energy being absorbed must equal the energy required to make the jump.
The energy of a photon is tied to the wavelength of the energy being absorbed, and this energy must match the energy required to move the electron from the low energy orbit to the higher energy orbits. When an atom or molecule has an electron out of it's normal orbit in this manner it is said to be in an excited state. We all know that excited states normally have short lifes and the atom or molecule will emit a photon of it's own to bring the excited electron back to its normal state. The energy, or wavelength, will depend on which jump the electron takes to make it's way back to the normal state.
Because of this, the energy released comes out in peaks when plotted against the wavelength. This behaviour, or property, is called the emissivity of the compound. Emissivity varies from 0 to 1, meaning it can vary from no energy being absorbed or emitted up to 100% of the energy being absorbed or emitted. The graphs below show the emissivity of both water vapor and carbon dioxide plotted against the wavelength of energy, either absorbed or released.
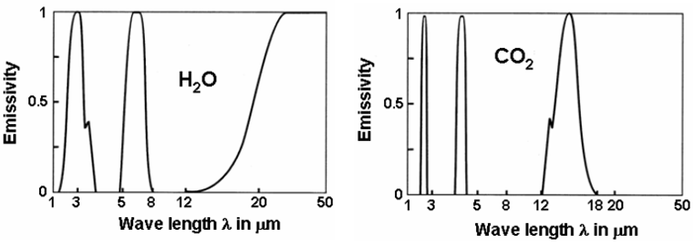
Notice the isolated peaks in the emissivity graphs indicating the jumps of the electrons from one energy level to another. The values of wavelengths between 1μm and 50μm is in the infrared band of energy. Normal earth temperatures emit energy centered around 10μm but the emissions cover the spectrum between 1μm and 50μm.
If we overlay the two graphs, we get the graph to the right. Remember the peak energy emissions for the earth are in the 10μm range where there is no absorption. Also look at the carbon dioxide spike between 1μm and 3μm, that overlays with a similar absorption band with water vapor. Since there is much more water vapor in the atmosphere than carbon dioxide, this band will do nothing to add to global warming.
Below is a graph showing the distribution of energy from the earth when the surface is at 0°F and 100°F. This distribution is based on Planck's equation. These two temperatures were chosen because they encompass most of the normal temperatures on earth.
Notice that at higher temperatures the area between 8μm and 12μm (where there is no absorption by water vapor or carbon dioxide) is larger than at colder temperatures. Doing some numerical integration on this curve shows that at 100°F 40% of the energy emitted by the earth passes freely back through the atmosphere while at 0°F it's only 33%.
In other words, at colder temperatures the greenhouse effect works to save more energy to warm things back up and at higher temperature more energy is released to cool it down. My hope here is to provide some science information without getting deep into a bunch of equations to help you better determine the validity of some of the claims made in the area of global warming. If there is something in any of these post you have questions on, please ask. Depending on the complexity of the question, I can try to answer them in a reply or in a separate post.
Related posts:
Man Made Global Warming
Are Plants Starving for Carbon Dioxide
Greenhouse Effect
Carbon Dioxide Saturation in the Atmosphere
Explaining the Earth's Albedo
I’m glad I ran across your blog. I’ve read through your ‘related posts’. I’ve never read anything that explained so simply the actual science behind this debate. Thank you!
Most of this is science but a lot of this is common sense. If people know a little of the science they can see through the haze of what is given to us by the media. Thank you for your support and I am glad I could help.