Everyday Geography #4 Why some dry soils crack
It might already have caught your eye that during a dry and warm period cracks appear in some soils, and if it didn't, you probably won't be surprised if I told you it does. But this does not happen in every soil, as an example we do not find these cracks on sandy beaches. Why certain soils show these cracks might be interesting for curious people like you to know.

What causes the cracks?
The occurrence of cracks is mostly caused by the composition of the soils, to be specific, the fraction of clay. Clays have the unique ability to absorb water into their mineral structure, making them swell when wet. Why this is exactly will be explained later. During a wetter period, the clays have absorbed the water and expanded. Since the soil is surrounded by other soils or obstacles, it has no choice but to expand in the height. The clay in the soil expands, taking up a larger volume as it would without water. When afterwards this soil dries again, water is removed from the clay particles and they shrink. Now there are no limitations in what direction to shrink so the volume decreases in all directions. This causes cracks to form in the soil.
Clay sediments can be defined as grains of a certain size (smaller than 2µm), or as grains of a certain mineral depending on the field you work in. The swelling capacity is not affected by the size of the grain, but is strongly defined by the mineral that makes up the particle.
Why do clays expand?
Fine, we know that some clays expand when wet, and that this expansion causes cracks when the soil dries again, but we still haven't answered why these clays expand. Clay minerals are generally made up out of layers with tetrahedral silicates, octahedral metal oxides (mostly Al, Mg and Fe), and layers of exchangeable cations connecting the different molecular layers.
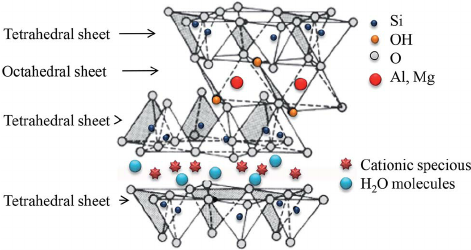
Image source (clay mineral smectite)
This layer of exchangeable cations is what allows clay to swell. Cations have a strong positive charge that holds negative tetrahedral sheets together. The only thing is that the cations attract water more strongly than the tetrahedral sheets. This causes water molecules to penetrate the spaces between layers pushing them apart since H2O is larger than an individual ion. Depending on the composition of the clay mineral, the capacity to swell and shrink changes.
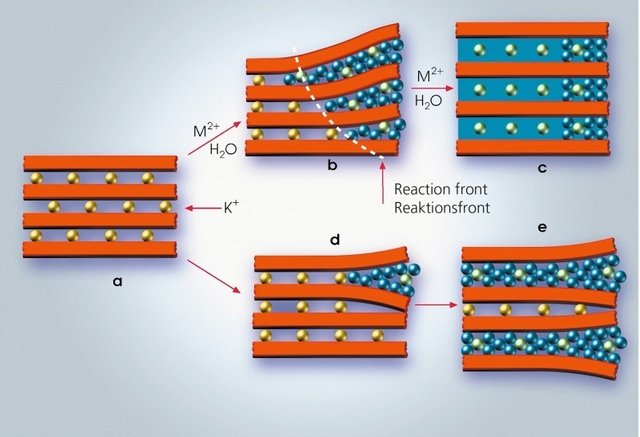
Conclusion
Next time you see a cracked soil in the summer you know exactly what is going on, even on the molecular level! Have you seen this often where you live or would you like to add something? I'd love to hear it! If you like these posts of Everyday Geography, make sure to follow me or check out my previous posts.
Sources
Why Clays Swell - Emiel J. M. Hensen and Berend Smit
Everyday Geography: previous posts
Everyday Geography #3 What makes salt tolerating vegetation special
Everyday Geography #2 Meandering, braided and straight rivers
Nice work, please do keep it up.
Thank you! I really do my best to post interesting articles and I appreciate the feedback
@originalworks
The @OriginalWorks bot has determined this post by @samve to be original material and upvoted it!
To call @OriginalWorks, simply reply to any post with @originalworks or !originalworks in your message!