Sample Review Problems on Specific Heats, Internal Energy, Enthalpy and Entropy
Hi folks!
Well, thermodynamics is a very great subject for students who are about take up and those who are currently taking up. The good thing about this subject is that you can always connect it to other problems (let say gases and the interesting topic regarding steam and its cycles) since the basic concepts are applied over and over again.
Today, allow me to share a problem wherein it showcases the thermodynamic properties being exhibited by ideal gases and these properties are as follows: Specific Heat Capacity both at constant volume (Cv) and at constant pressure (Cp), Internal Energy (U) and lastly, Enthalphy (H).
So without further ado, let me start with this first sample review problem and I’m pretty sure you’ll get excited about it and I will show the easiest way in solving for we know there are many ways in killing a cat and does in solving mathematical problems too.
A gas initially at 15 psia and 2 ft3 undergoes a process to 90 psia and 0.60 ft3, during which the enthalpy increases by 15.5 BTU; Cv = 2.44 BTU per lbm per unit degree Rankine. Determine the following:
- Change in Internal Energy
- Specific Heat Capacity at Constant Pressure (Cp)
- Gas Constant (R)
So let’s start solving this problem.
Change in Internal Energy
Since we are provided with the change in enthalpy (delta H) being experienced by the gas, we can directly obtain the change in internal energy (delta U) by using the formula in computing for the enthalpy and the same time we are provided with both the initial and final states of the gas wherein the pressure (P) and volume (V) are explicitly stated. And the calculation goes like this:
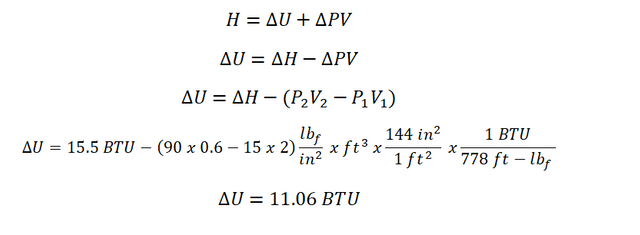
And therefore, the change in internal energy is equal to 11.06 BTU.Specific Heat Capacity at Constant Pressure (Cp)
As for this one, the easiest way in solving this problem is remembering the relation of both the specific heat capacity at constant volume (Cv) and at constant pressure (Cp) which is also connected to the relation of both the change in enthalpy (delta H) and change in internal energy (delta U). So here is my calculation for this question:
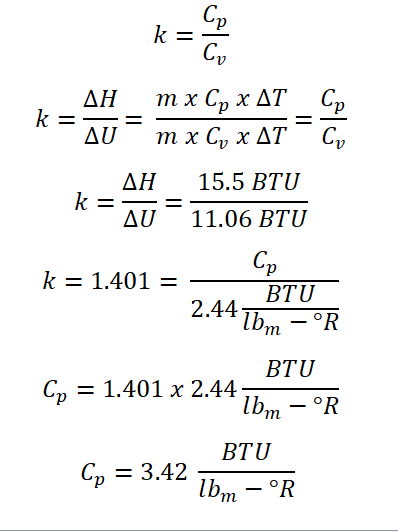
Based on the above photo, I simply use both the change in enthalpy and change in internal energy for the very reason that change in enthalpy utilizes the specific heat capacity at constant pressure (Cp) and whereas for the change in internal energy it utilizes the specific heat capacity at constant volume (Cv), and thus since both are experiencing temperature change, we can simply cancel it out and the same happens also with the mass. And therefore the specific heat capacity at constant pressure (Cp) is equal to 3.42 BTU per lbm per unit degree Rankine.Gas Constant (R)
For this problem, we will just be using the relations for the gas constant (R), the specific heat capacity at constant volume (Cv) and at constant pressure (Cp). And the solution goes like this;
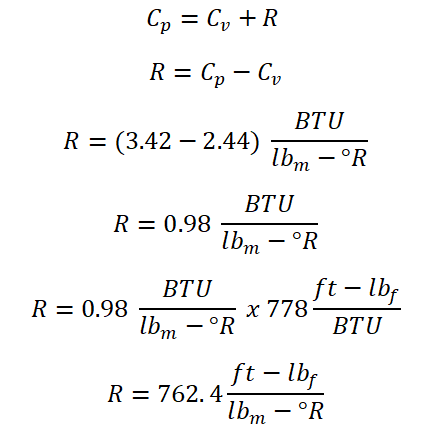
As you've noticed above, the gas constant is having two units both of which are in English system of units, the very reason for that is as far as I can remember, the gas constant is always presented in units of ft-lbf per unit lbm per unit degree Rankine and whereas for the specific heat capacities whether it is at constant volume (Cv) or at constant pressure (Cp) is always expressed in units of BTU per unit lbm per unit degree Rankine. And with that we 0.98 BTU per unit lbm per unit degree Rankine as the gas constant of the gas being used in this problem wherein it is also equal to 762.4 ft-lbf per unit lbm per unit degree Rankine.
So I guess that would be all for this sample review problem on specific heats, internal energy and enthalpy.
Thank you for spending your time reading this blog post of mine.
Much love and respect.
Ace | @josephace135
Reference:
- Hipolito B. Sta. Maria, Thermodynamics
Solutions are being made by me and the sample review problem is found in page 50, problem number 9.
Presentation of equations and formulas were made possible by MS Word 2010 Equations function.
Screenshots were made possible by utilization of Snipping Tool application.
Your blog has received an upvote from the communal account of Steemph.antipolo for being an active discord member and as an active community member. Keep up the good work and best of regards. Keep on Steeming!
You can get a support by joining our discord channel and gain votes from
our curators. Join our discord now
https://discord.gg/7w3hJqw
If you would like to support steemph.antipolo project you can help by delegating your spare SP to us, just click the link below.
50 SP 100 SP 200 SP 300 SP 400 SP 500 SP 1000 SP
Thermodynamics the terror of many university, more than all those who study chemistry, haha thank you for remembering one of my traumas. Haha
Well I understand bro, we are all unique. I guess you have your own forte too.
Btw, its a must for mechanical engineering students, they need to master this one out since this is just basic. Hahahahaha if time permits hopefully I can share some of the basics and to the challenging topics derived from thermodynamics. :)
You have been upvoted by the @sndbox-alpha! Our curation team is currently formed by @jeffbernst, @bitrocker2020, @jrswab & @teachblogger . We are seeking posts of the highest quality and we deem your endeavour as one of them. If you want to get to know more, feel free to check our blog.
Congratulations! This post has been upvoted by the communal account, @steemph.cebu by josephace135 being run at Teenvestors Cebu (Road to Financial Freedom Channel). This service is exclusive to Steemians following the Steemph.cebu trail at Steemauto. Thank you for following Steemph.cebu curation trail!
Don't forget to join Steem PH Discord Server, our Discord Server for Philippines.