Ideal Gas: Specific Heats, Internal Energy, Enthalpy and Entropy
Hi folks!
Today allow me to continue sharing about the Ideal Gases wherein I will elaborate the other important parameters wherein gases are taken into account and these are as follows:
- Specific Heat (c)
- Internal Energy (U)
- Enthalpy (H)
- Entropy (S)
So without further ado, I will now start my discussion regarding these four parameters.
SPECIFIC HEAT (c)
The specific heat or usually known as specific heat capacity of the substance is the amount of heat that is required to change the temperature of unit mass of one degree. Specific heat capacity of a substance like gases is expressed in units of:
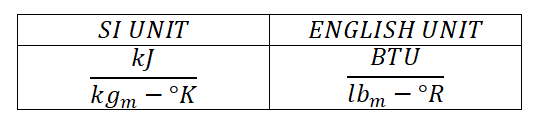
Take note that 1 British Thermal Unit (BTU) is equal to 778 ft-lbf.
Actually there are two specific heat capacities one wherein it is at constant volume (Cv) and whereas the other one is at constant pressure (Cp). Furthermore, the specific heat capacity at constant volume (Cv) and specific heat capacity at constant pressure (Cp) are in close relation wherein it takes into account the gas constant (R) and the adiabatic index (k) of a certain ideal gas.
So here are the relations for the specific heat capacity at constant volume (Cv) and at constant pressure (Cp).
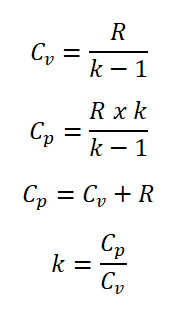
We must always take note that the value of the adiabatic index (k) must always be greater than one. For example, air it has k = 1.3 for hot-standard and k = 1.4 for cold-standard, respectively.
INTERNAL ENERGY (U)
Internal energy (U) is the total energy in a system which includes both the potential and kinetic energy and is associated with the random and disordered motions of the molecules. Internal energy is often calculated as a function of the specific heat capacity at constant volume and the temperature change being experience by the substance. And here is the formula for calculating the internal energy (U) and as for me, I use a mnemonic in remembering easily the formula in finding the internal energy and that is with the initials UV wherein I always say it as “University of Visayas” which is one of the universities here in Cebu, there are many ways of mastering it and making mnemonics, and the one I told is my own way of remembering the formula.
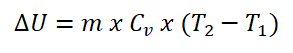
This thermodynamic property is often expressed in the units of:
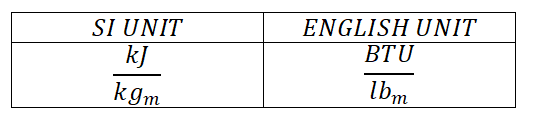
ENTHALPY (H)
Enthalpy is expressed as the summation of the internal energy and the product of both the pressure (P) and volume (V). For ideal gases operating at constant pressure, enthalpy is expressed as the product of the change in temperature and the gas specific heat capacity at constant pressure.
And here is the formula in computing for the enthalpy (H) of an ideal gas and by the way, the same as for internal energy I use a mnemonic in remembering the formula and my mnemonic is HP wherein I will just think that it is Hewlett Packard which is a well-known tech company especially in computers and printers, and I used it as the initials of that company which has been proven to help me in remembering the formula for enthalpy.
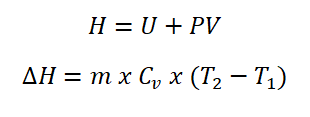
This thermodynamic property is often expressed in the units of:

ENTROPY (S)
Entropy (S) is often referred to us as the degree of randomness of a substance. Entropy is expressed in the following differential equation:
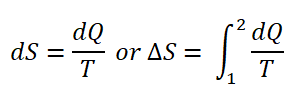
As you’ve noticed there is a dQ, it is actually the heat transferred at the temperature (either at constant volume or constant pressure) which can be further simplified as and be integrated;
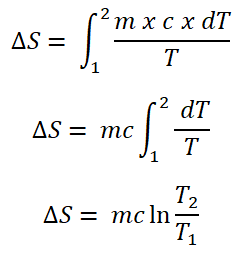
Take note that m denotes as the unit mass which is expressed in units of lbm in English system of units and kgm in SI units; whereas, c denotes that specific heat capacity.
And here are the formulas for the entropy for gases operating either in constant volume (V) or constant pressure (P).
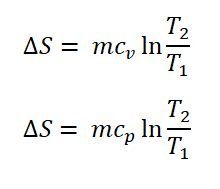
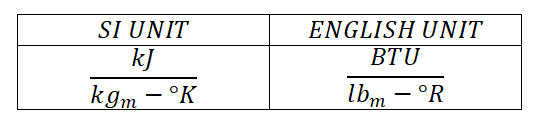
And this thermodynamic property is expressed in the units of:
As for entropy, I just remember the differential equation that it was derived from and I just take note of adiabatic index (k) and gas constant of a gas (R) whenever I meet problems wherein Cv and Cp weren’t provided. Although most of the problems relating to entropy are provided with the values for Cv and Cp.
I guess that would be all for this blog post of mine regarding ideal gases’ specific heat capacities, internal energy, enthalpy and entropy.
Thank you for spending your precious time reading this blog.
Much love and respect.
Ace | @josephace135
Reference:
- Hipolito B. Sta. Maria, Thermodynamics
Your blog has received an upvote from the communal account of Steemph.antipolo for being an active discord member and as an active community member. Keep up the good work and best of regards. Keep on Steeming!
You can get a support by joining our discord channel and gain votes from
our curators. Join our discord now
https://discord.gg/7w3hJqw
If you would like to support steemph.antipolo project you can help by delegating your spare SP to us, just click the link below.
50 SP 100 SP 200 SP 300 SP 400 SP 500 SP 1000 SP
You have been upvoted by the @sndbox-alpha! Our curation team is currently formed by @jeffbernst, @bitrocker2020, @jrswab & @teachblogger . We are seeking posts of the highest quality and we deem your endeavour as one of them. If you want to get to know more, feel free to check our blog.
Congratulations! This post has been upvoted by the communal account, @steemph.cebu by josephace135 being run at Teenvestors Cebu (Road to Financial Freedom Channel). This service is exclusive to Steemians following the Steemph.cebu trail at Steemauto. Thank you for following Steemph.cebu curation trail!
Don't forget to join Steem PH Discord Server, our Discord Server for Philippines.
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by josephace135 from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.
This post make my nose bleed but it's very informative. Thanks for sharing!