IDEAL GAS LAWS: Sample Problems with Explanations
Hi folks!
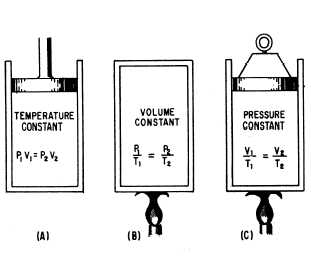
Today allow me to share some sample review problems wherein the Ideal Gas Laws are being applied. For the whole duration of this blog we will be meeting terminologies like "tanks" (not the one being used by the military wherein it is used to bombard enemy positions using its big guns) and "tire". And you might be wondering those terminologies are being used. Well the very reason for that is due to the fact the some gases need to be stored and be maintained at a certain pressure. These "tires" and "tanks" are play an important role in proving the behavior of fluids like gases, wherein gases take the shape of the container that they are enclosed or stored and follow their volume though and that is a great manifestation that the atoms/molecules of gases are separated apart from each other and that is the very reason why gases especially those that are flammable are stored and maintained under operating pressures.
So let me start solving this first problem that I am going to solve and explain.
A 10 ft3 tank contains gas at a pressure of 500 psia, temperature of 85 degree Fahrenheit and a weight of 25 pounds. A part of the gas was discharged and the temperature and pressure changed to 70 degree Fahrenheit and 300 psia, respectively. Heat was applied and the temperature was back to 85 degree Fahrenheit. Find the final weight, volume, and the pressure of the gas.
So based on the aforementioned problem we are given with the following parameters:
| Parameter | State 1 | State 2 | State 3 |
|---|---|---|---|
| Pressure (P) | 500 psia | 300 psia | ? |
| Temperature (T) | 85 degree Fahrenheit | 70 degree Fahrenheit | 85 degree Fahrenheit |
| mass (m) | 25 lbm | ? | ? |
The solution for this sample review problem goes like this;
We all know that gases occupy and follow the shape of the container they are being stored or enclosed, therefore the volume of the container they are being stored is just equal to the volume of the gas from its initial state (State 1) up to the final state (State 3). And the keyword for this sample review problem is the word TANK.
Therefore,
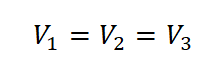
Hence, we can now start solving for the following:
- Final weight, m3
As for me, I will be using the universal gas constant formula wherein we all know that the volume from the initial state up to the final state is equal and I will be equating the general gas constant equation for me to make the formula that it is looking for the volume (V) as seen in the derivation I made in the photo below.

And then, using that derived formula;
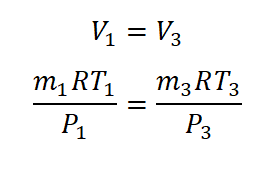
And since the temperature in the initial state (T1) and final state (T1) are the same, both of them would be cancelled out and although we don't have any idea on the gas constant of the gas (R), it will also be cancelled since it will be the same gas constant that would be used from initial state up to the final state. So we arrive with a derived formula of;

As you've noticed we don't have the value of the absolute pressure at the final state (P3). so we need to obtain it first. And since the volume is constant, we will be using Gay-Lussac's Law in obtaining the pressure at the final state.
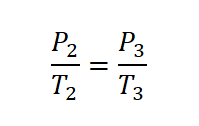
And then, we obtained the pressure at the final state (P) which is 308.5 psia.
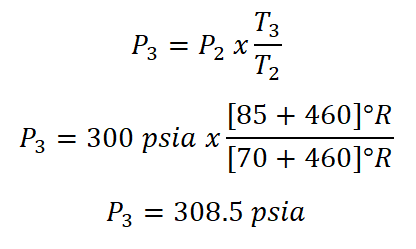
Finally, we can now obtain the final weight of the gas by substituting the final absolute pressure to the previous equation derived. And the calculation goes like this,

And the final weight of the gas (m3) is 15.43 lbm. - Final volume, V3
Like I've mentioned before I started solving the problem, the keyword is tank. And therefore, the volume is equal from the initial state up to the final state.

- Final Pressure of the gas, P3
Since I have obtained the final pressure of the gas (P3) before I have obtained the final weight of the gas (m3).
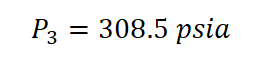
Second and last problem would be this one,
If 100 ft3 of atmospheric air at zero Fahrenheit temperature are compressed to a volume of 1 ft3 at a temperature of 200 degree Fahrenheit, what will be the absolute pressure of the air in psi (pound force per square inch)?
In this problem, we are given with the following parameters:
| Parameter | State 1 | State 2 |
|---|---|---|
| Pressure (P) | 14.7 psia | ? |
| Volume (V) | 100 ft3 | 1 ft3 |
| Temperature (T) | 0 degree Fahrenheit | 200 degree Fahrenheit |
As you've notice I placed 14.7 psia in State 1 wherein we didn't find any mention of 14.7 psia in the problem, but the very reason why I placed 14.7 psia is simply because of the keyword atmospheric air, wherein it has an absolute pressure of 14.7 psia.
Moving forward in solving this problem, we will be using the Combined Gas Law formula in order to obtain the pressure wherein it experience compression.

And here are the calculations;
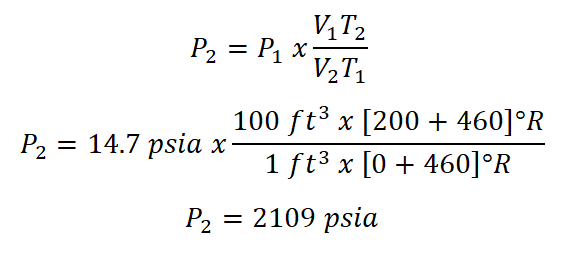
Therefore, the pressure of the air after it was compressed is 2109 psia.
And so I was able to present and solve two problems that used the ideal gas law and its formula and I guess that would be all for this blog of mine.
Thank you for spending your time reading this blog.
Much love and respect.
Ace | @josephace135
Reference:
- Thermodynamics by Hipolito B. Sta. Maria
Note:
All the solutions presented in this article are made by me and the presentation of formulas and calculations were made possible by Microsoft Word equation tools and the screenshots are obtained thru the usage of Snipping Tool.
Your blog has received an upvote from the communal account of Steemph.antipolo for being an active discord member and as an active community member. Keep up the good work and best of regards. Keep on Steeming!
You can get a support by joining our discord channel and gain votes from
our curators. Join our discord now
https://discord.gg/7w3hJqw
If you would like to support steemph.antipolo project you can help by delegating your spare SP to us, just click the link below.
50 SP 100 SP 200 SP 300 SP 400 SP 500 SP 1000 SP
You have been upvoted by the @sndbox-alpha! Our curation team is currently formed by @jeffbernst, @bitrocker2020, @jrswab & @teachblogger . We are seeking posts of the highest quality and we deem your endeavour as one of them. If you want to get to know more, feel free to check our blog.
Congratulations! This post has been upvoted by the communal account, @steemph.cebu by josephace135 being run at Teenvestors Cebu (Road to Financial Freedom Channel). This service is exclusive to Steemians following the Steemph.cebu trail at Steemauto. Thank you for following Steemph.cebu curation trail!
Don't forget to join Steem PH Discord Server, our Discord Server for Philippines.