It is possible to obtain a temperature below absolute zero
Image source: [1]
If we consider the definition of Temperature as "that which can be measured with a thermometer".
This definition implies that Temperature is the absolute value of the mean velocity of the particles that make up a system, so there can be no negative mean absolute value.
According to this definition the temperature could be 0, but if there were a process that would bring us to the absolute 0 in a finite number of steps we would lead to some contradictions with what we observed experimentally on specific heats (which would be 0 and that contradicts with what That we know of them).
This postulate is known as the Third Principle of Thermodynamics and as all principles in science (such as "all crows are black") is valid because we have not yet observed any experimental fact that contradicts it (for example find a green crow Or white).
However, today we know something more about nature than when this principle was formulated at the beginning of the last century. We know that at the atomic level there is no energy level 0 since there is a non-zero fundamental level with a certain energy and velocity.
Now, thermodynamics has an entropy definition that is
Therefore, we have a definition of temperature that is the variation of Heat divided by the variation of entropy of a system
The variations of heat and of entropy yes that have sign, therefore we could get to have negative temperatures.
Here the link for more details: [2]
Here an explanatory video:
Video credits: Pocket Science

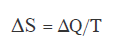
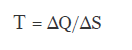

referring to the headline only.
the answer is NOPE.
by definition Absolute Zero is an absolute.
can't get no colder than that.
unless the definition is changed.
I was wrong, I did not even notice it, I'll be more careful for the next post
like I said. I din't read the post...only the headline. I'm skimming JUST before I hit the sack.