Introduction to astronomy #3
Hello friends, continuing with the previous posts [1] - [2] about some basic concepts in astronomy.
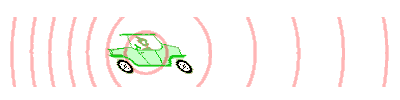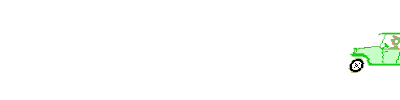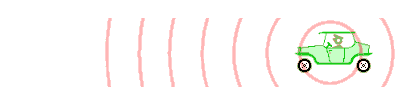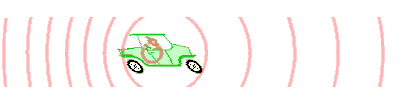
Doppler effect: The Doppler effect (or the Doppler shift) is the change in frequency or wavelength of a wave for an observer who is moving relative to the wave source.
Image source [1]: An animation illustrating how the Doppler effect causes a car engine or siren to sound higher in pitch when it is approaching than when it is receding. The pink circles represent sound waves.
The Doppler effect for electromagnetic waves such as light is very useful in astronomy. It has been used to measure the speed at which stars and galaxies approach or recede; that is, its radial velocities or to detect exoplanets.
Spectral line: is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from emission or absorption of light in a narrow frequency range, compared with the frequency frequencies. Spectral lines are often used to identify atoms and molecules.
Image source [2]: Continuous spectrum.
Image source [3]: Emission lines.
Image source [4]: Absorption lines.
Spectrum: is a condition that is not limited to a specific set of values but can vary, without steps, across a continuum.
The spectra show, in addition to different intensities in the colors, also some brighter lines and other darker lines that are precisely the so-called spectral lines.
Spectral type: In the Universe there is a great diversity of stars, the most massive have up to a hundred times the mass of the Sun, while the less massive ones have only a few tenths of the mass of the Sun. Also the range of temperatures varies greatly, from stars with temperatures of 50,000 degrees K (Kelvin) on its surface to stars with temperatures of 1000 K, also on its surface.
The temperature of a star determines the ionization states of the different chemical elements it has. The energy levels at which the ions of a given star are determined determine the frequencies of the emission or absorption spectral lines of said star, which in general are different for different temperatures.
State of matter: The matter is constituted by particles. In a system of particles, the way in which they are added together determines the state of said system, which can be solid, liquid or gaseous.
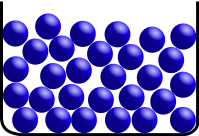
Solid: In a solid, constituent particles (ions, atoms, or molecules) are closely packed together. The forces between particles are so strong that the particles can not move freely but can only vibrate. As a result, a solid has a stable, definite shape, and a definite volume. Solids can only change their shape by force, as when broken or cut.
Image source [5]: A crystalline solid: atomic resolution image of strontium titanate. Brighter atoms are Sr and darker ones are Ti.
Liquid: A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure.
Image source [6]: Structure of a classical monatomic liquid. Atoms have many nearest neighbors in contact, yet no long-range order is present.
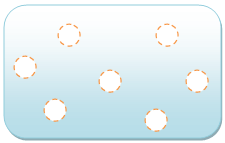
Gas: A gas is a compressible fluid. Not only will gas conform to the shape of its container but it will also expand to fill the container.
Image source [7]: The spaces between gas molecules are very big. Gas molecules have very weak or no bonds at all. The molecules in "gas" can move freely and fast.
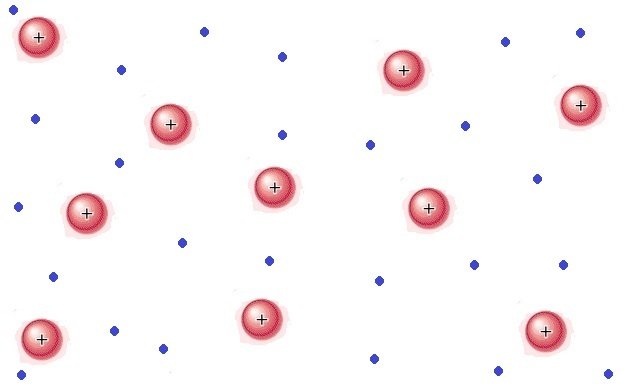
Plasma: Like a gas, plasma does not have a definite shape or volume. Unlike gases, plasmas are electrically conductive, it produces magnetic fields and electric currents, and responds strongly to electromagnetic forces. Positively charged nuclei swim in a "sea" of freely-moving disassociated electrons, similar to the way such charges exist in conductive metal, where this electron "is" allows matter in the plasma state to conduct electricity.
Image source [8]: In a plasma, electrons are ripped away from their nuclei, forming an electron "sea". This gives it the ability to conduct electricity.
Movements of the particles and temperature concepts:
The atoms and molecules that make up a system have an energy that makes them be in constant motion. To this energy of the system a variable has been associated to which we call temperature.
The constant internal movement in a gas, produces collisions of the particles against the walls of the container that contains it. The pressure of a gas towards the walls of said container is a result of the amount and speed of the collisions between the particles. That is, said pressure depends on the amount of particles as well as their kinetic energy.






Really nice...
Reminds me of my physics class back then in high school
Nice one
Very good
Thanks :D
The stars are our ancestors;s; we are star dust: it is one of the great discoveries of contemporary astronomy