How do Snowflakes form such Beautiful Patterns?
Ever heard of the saying that all snowflakes are created different? Regardless whether that is true or not, which I will discuss, they sure are one of nature’s most beautiful bits of art. How do they form, where do they form and how do they manage to look so incredible?
Snowflake Formation
The birth of a snowflake begins high up in Earth’s atmosphere. At such altitudes, water is in the form of water vapour that eventually falls as rain, snow or hail, and so on. How can a bit of water become such a work of art?
The Foundation of a Snowflake – A particle
Believe it or not, for a snowflake to begin to grow, it needs a particle; in other words, a seed [1]. These particles could either be little dust grains or pollen particles. Water vapour high up in the atmosphere clings onto said particle and coats it. Under the right conditions, i.e. temperature and humidity, this water vapour freezes, and so this structure becomes a tiny crystal of ice. Provided that the particle is of sufficient size, the ice that coats it forms a hexagonal structure - we now have the birth of a snowflake.
The Structure of a Water Molecule
Water’s chemical structure is H2O; it is composed of one Oxygen atom and 2 Hydrogen atoms. The angle between the 2 Hydrogen atoms is about 104.5 degrees [2]. As a result, water forms hexagonal structures when it is frozen; hence why all snowflakes have 6 arms, or 6 sides.

Figure 1: An image showing a water molecule. The red ball is the Oxygen atom and the white balls are the Hydrogen atoms
The Growth of a Snowflake
Since this newly formed particle is now covered in ice, it becomes heavier than the particles around it; consequently, it begins to fall [3]. As it falls, more and more water vapour in the atmosphere clings onto this ice crystal and freezes. The hexagonal arrangement for frozen water is maintained due to the chemical structure of water, as mentioned above; hence why snowflakes always have 6 distinct sides. The further and further down the snowflake falls, the bigger it becomes.
Are all Snowflakes different?
The simple answer is yes. By having different combinations of temperature and humidity in the atmosphere, water vapour freezes onto the crystal in different ways. Some conditions allow snowflakes to grow arms, and have arms growing out of those arms - others form columns of ice around the ice structure, and so on. As the combination of temperature and humidity changes whilst this snowflake falls towards the ground, the way water vapour freezes onto it changes; this gives rise to endless possibilities of shapes the snowflake can form. This explains the beautiful pattern and variation in every single snowflake.
Still confused?
You may be thinking there should be at least one snowflake that is the same as another... well, if you consider that for every snowflake there are millions and millions of atoms making it up, then you will be able to see the probability that every single atom is arranged the same way in one snowflake to another is almost impossible!
Is all Snow made up of Snowflakes?
The form that snow reaches the ground is largely dependent on the conditions of the atmosphere between the formation of the seed, and the ground [1]; let me explain. If the entire atmosphere from top to bottom is suitably cold and humid for snowflake formation, then snow hits the ground as snowflakes; as shown in figure 2. What if we have unexpected columns of hot air – i.e. heat that is too hot for snowflakes to remain frozen?
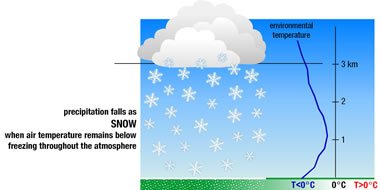
Figure 2: Image showing how Snowflakes reach the ground (by NOAA)
Sleet
As you can see from figure 3; a shallow layer of warm air in the atmosphere causes partial melting of the snowflakes. When they exit this part of the atmosphere back into a deep layer of cold air, they re-freeze and reach the ground as Sleet.
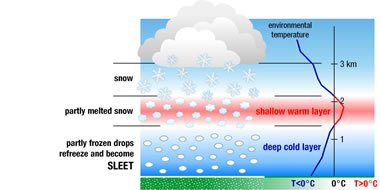
Figure 3: Image showing how Sleet reaches the ground (by NOAA)
Frozen Rain
As you can see from figure 4; a deeper layer of warm air in the atmosphere causes the snowflakes to melt completely. When they exit this part of the atmosphere back into a shallower layer of cold air, they become what is known as Freezing Rain. The concept of Freezing Rain is quite interesting. Due to the water passing through such a shallow column of cold air, it becomes supercooled. This means that it is liquid when it is falling, but when it hits something (for example, the floor), it reacts and becomes clear ice instantly. The video below shows how you can try this at home; it is in fact, super-cool!
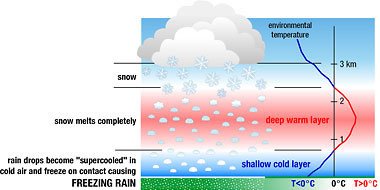
Figure 4: Image showing how Freezing Rain reaches the ground (by NOAA)
Here is your demonstration of supercooling liquids, at home!
This video will further explain in depth Snowflake formation and how they really are an intricate piece of art!
If you have any questions, leave them below and until next time, take care.
~ Mystifact
References:
[1]: http://geology.com/articles/snowflakes/
[2]: https://en.wikibooks.org/wiki/Structural_Biochemistry/Water
[3]: https://www.popsci.com/different-snowflake-shapes
Please note; no copyright infringement is intended. All images used have been labelled for re-use on Google Images. If any artist or designer has any issues with any of the content used in this article, please don’t hesitate to contact me to correct the issue.
Relevant articles:
Solids, Liquids and Gases... Is that it? - Part 2
Solids, Liquids and Gases... Is that it? - Part 1
What happens if you are STRUCK by Lightning?
Previous articles:
How Masturbation affects your Confidence
Everything you need to know about the Solar Eclipse!
Our Solar System, Our Home
Follow me on: Facebook, Twitter and Instagram, and be sure to subscribe to my website!


I just have been upvoted you because I like and enjoyed your post! keep publishing interesting and informative articles. If you wish you can check my post iamjeydii. Follow me @iamjeydii
Its incredible how no 2 snowflakes r d same...