Simple Diatillation
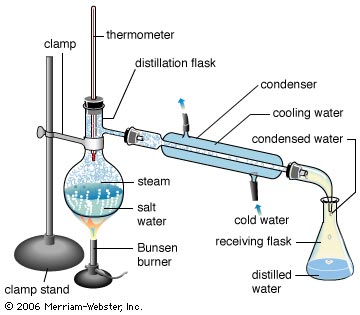
Distillation
Simple distillation is a procedure by which two liquids with different boiling points can be separated. Simple distillation (the procedure outlined below) can be used effectively to separate liquids that have at least fifty degrees difference in their boiling points. As the liquid being distilled is heated, the vapors that form will be richest in the component of the mixture that boils at the lowest temperature. Purified compounds will boil, and thus turn into vapors, over a relatively small temperature range (2 or 3°C); by carefully watching the temperature in the distillation flask, it is possible to affect a reasonably good separation. As distillation progresses, the concentration of the lowest boiling component will steadily decrease. Eventually the temperature within the apparatus will begin to change; a pure compound is no longer being distilled. The temperature will continue to increase until the boiling point of the next-lowest-boiling compound is approached. When the temperature again stabilizes, another pure fraction of the distillate can be collected. This fraction of distillate will be primarily the compound that boils at the second lowest temperature. This process can be repeated until all the fractions of the original mixture have been separated.
Source: http://www.wiredchemist.com/chemistry/instructional/laboratory-tutorials/distillation
Destillation???
ja, was willst du denn destillieren?
he wants to destillate milk.... and she....
und sie will sahne.... haha