Increased Accuracy During the Identification of THC and CBN using Proton Nuclear Magnetic Resonance Spectroscopy Analysis
Experiments and analyses have produced useful data for interpretation. Solutions which allow more precise identification, further benchmarking of THC and CBN containing samples.
The following are Proton Nuclear Magnetic Resonance (1H-NMR) Spectroscopy Analysis plottings produced at the University of Ottawa facilities. Sample analysis was conducted by Glenn A. Facey, Ph.D. (Gérant du Laboratoire RMN | NMR Facility Manager)
Sample preparation, data comparison and interpretation are completed by the composer of this document, test subject and inventor of technologies Dean Piecka.
The issue with analysis of phyto-synthesized compounds is the difficulty in isolating them without use of destructive sample preparation and analysis methods. Gas chromatography and distillation provide data though not without causing changes in the chemical structures of compounds. These analyses of phyto-synthesized compounds do solve the problems of chemical changes being accelerated by technique and source material choice. What is interesting is that we can add and subtract (mask) peaks corresponding with other expected molecules.
Figure 1. Proton Nuclear Magnetic Resonance Spectroscopy Analysis plotting of trichome heads isolated from stalks by application of the High-Voltage Field extraction method.
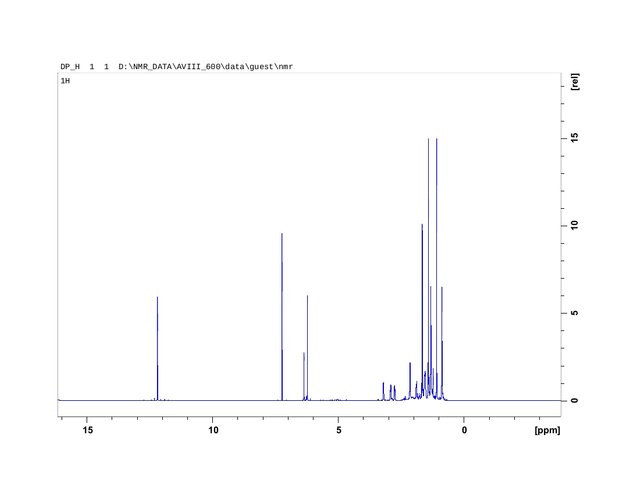
Figure 2. Proton Nuclear Magnetic Resonance Spectroscopy Analysis plotting of CBN concentrate produced from trichome heads (HVF extraction). Trichome heads applied as the source material increased isolation of oxidized hydrophillic non-psychopharmacologically active cannabinol (CBN) concentrate samples.
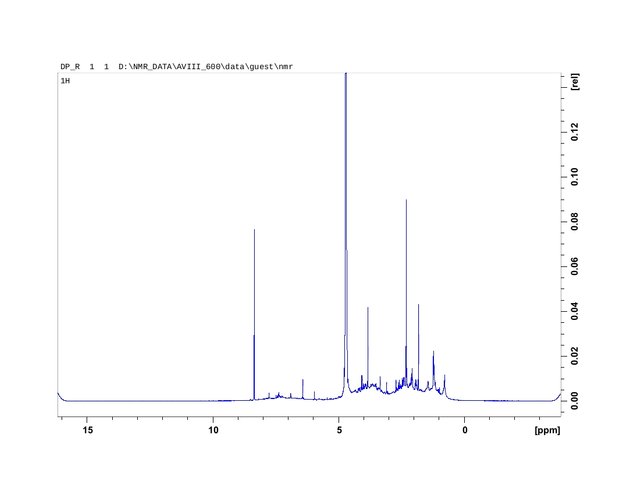
Figure 3. Comparison for differentiation of THC and CBN containing samples by their Proton Nuclear Magnetic Resonance Spectroscopy Analysis plottings.
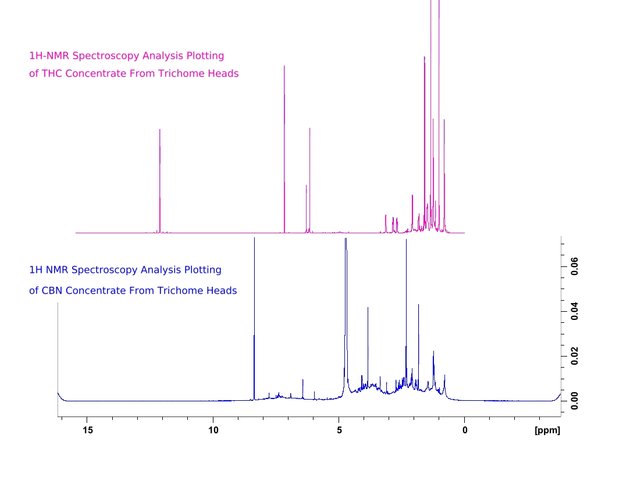
Figure 4. The identifying peaks of THC are encircled at 6.2 to 6.3 as well as the grouping between 0.9 and 1.7. Peaks which identify CBN (0.9 to 2.3) are now more accurately identified, due to the purity of trichome head source material used to further isolate samples. A plotting at 3.9 of varying intensities depending on the duration and intensity of oxidation in samples provided, have the greatest probability of being the presence of the degradation product of CBN (Cannabinol). There also exists the probability that this 3.9 plotting is the unique product of high-voltage electrolysis changing the molecular structures of CBN molecules to yield a new phyto-cannabinoid. The most likely probability is that I will confirm the electrolysis process to be an electric method of producing a phyto-cannabinoid which is found in tombs and ancient samples of cannabis. A degradation product of CBN. More study of datasets can confirm.
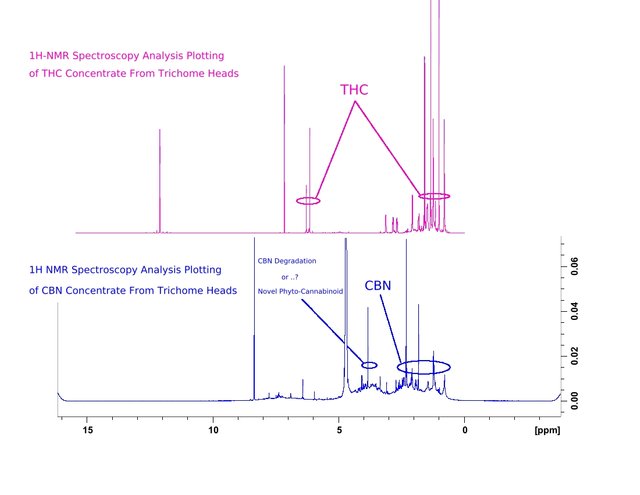
Figure 5. The superimposition of both THC and CBN Proton Nuclear Magnetic Resonance Spectroscopy Analysis plottings. For the purpose of visualisation we subtract the peak at 4.8 that identifies the deuterium solvent in which the hydrophilic sample is dissolved. Another peak exists at 7.2, chloroform solvent used to dissolve hydrophobic compounds may also be subtracted from the plotting.
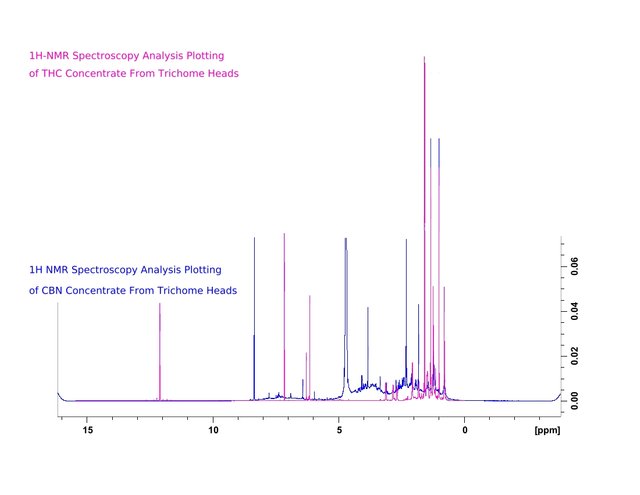
Figure 6. The superimposition of data from the previous figure has been coloured blue. This provides a composite that is very familiar to those who have analyzed cannabis samples by Proton Nuclear Magnetic Resonance Spectroscopy Analysis. Any samples which contain THC and CBN can be expected to have plottings similar to those of this composite.
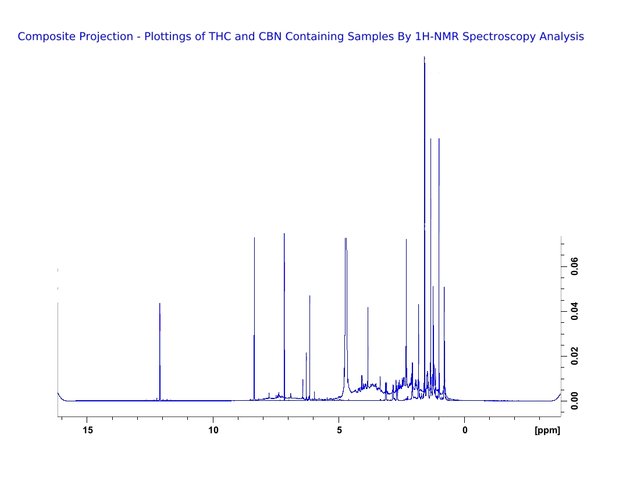
Figure 7. Does this look familiar? The combined plotting of THC and CBN was among the only publicly available data for comparison of phyto-cannabinoid samples analyzed by Proton Nuclear Magnetic Resonance Spectroscopy Analysis plotting. Now we can divide and recompose the inclusive plottings such as these below, by using more accurate samplings of THC and CBN from trichome heads.
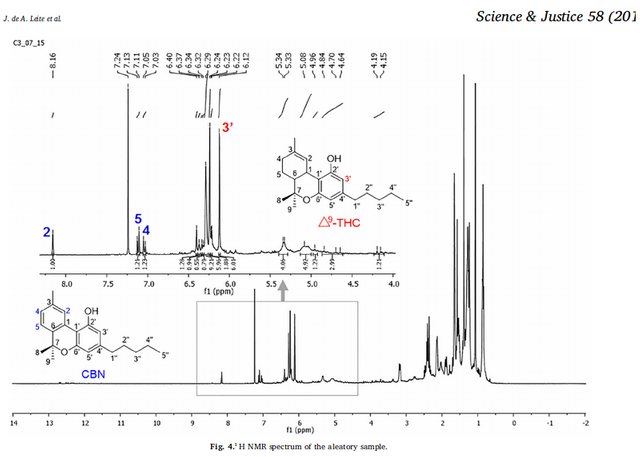
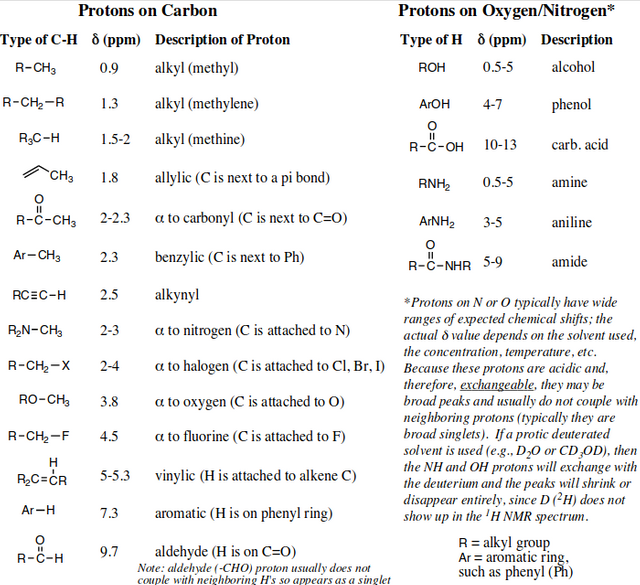
Figure 8. Here is a key to the plotting of chemical structures a molecule is comprised of. Several peaks may belong to the same compound within a sample analyzed. The chart indicates the accepted standard for identification of chemical component structures that constitute the entirety of the phyto-cannabinoid molecular structure.
Cannabinoid type one receptors should receive agonists as yellow concentrate of THC molecules (hydrophobic). Which are most effectively used by cannabinoid type one receptors at cell membranes within the brain where they are psychopharmacologically active.
The red CBN (hydrophilic) molecules are pharmacologically effective at cannabinoid type two receptor sites expressed by majority at the membranes of bone marrow cells. Cited are the properties of osteogenesis and apoptosis (cell death) of cancer cells. Cannabinoid type two receptors and the ligands which are pharmacologically effective in binding with them, are necessary elements of the immune system, contributing to the regeneration of immune cells which replenish blood from marrow-focused biosynthetic pathways.
Maybe we should not put the two molecules back together, then each can be administered to the correct end of the patient.
The links below are informal citations to confirmations of pharmacological properties demonstrated by the receptors and ligands of the endogenous cannabinoid system. It is quickly becoming the work of Immunologists as an immunological system rendered invisible by regulation which otherwise would have allowed the understanding and application of a biological system which causes apoptosis (cell death) of cancer cells in healthy immune systems.
The endogenous cannabinoid system is not less a part of the immune system than it's counterparts. Phagocytic cells which consume dead cells also metabolize them to produce "super-oxides" or ROS (reactive oxygen species)..these white blood cells have their origin among the various types of cells found in bone marrow. The some effects might also be parasympathetic by application of pharmacologically analogous phyto-cannabinoid agonists of cannabinoid type two receptors expressed by majority at the cell membranes of bone marrow cells.
https://www.ncbi.nlm.nih.gov/m/pubmed/17931597/
https://www.ncbi.nlm.nih.gov/pubmed/11269508
https://www.ncbi.nlm.nih.gov/m/pubmed/23103355/?i=6&from=/17931597/related
https://www.ncbi.nlm.nih.gov/m/pubmed/22038019/?i=4&from=/23103355/related