How Lead Acid Battery Works 🍎 Dead Battery Repair
Lead Accumulator Cell
The lead accumulator cell was constructed by French physicist Gaston Plante in 1859[1]. Shortly after this construction Camille Alphonse Faure amended it in 1881[2]. There is a container of glass or rigid rubber in which the dilute sulphuric acid of about 1.24 is filled. There are two lattice plates in the lead. These plates are called grids. Litharge (PbO) paste made in Dilute Sulfuric Acid in the grid's spaces is filled. Works on a grid Positive pole and second Negative pole. In some cells, the red Lead (Pb3O4) is filled in positive pole grid and filled in a litharge negative poll grid.
Chemical reactions before being charged
PbO + H2SO4 → PbSO4 + H2O
In this way, both grids have a mixture of PbO and PbSO4 initially.
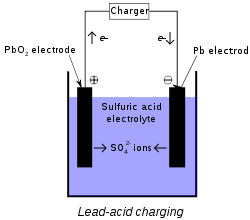
Charging Process: To charge the battery, the direct current flows from the external source. Battery chargers are used for this. The positive plate of the cell connects with the positive pole of the battery charger and the negative plate to the negative pole of the charger. When there is an electrical current in the battery, then there is the electrical decomposition of acidic water.
H2O → 2H+ + O-2
Hydrogen ion (H+) move toward negative plate and Oxygen ion (O-2) toward positive plate.
Chemical Reaction is as Follows:-
Positive Plate Reaction
PbO + O2- → PbO2 + 2e-
PbSO4 + O2- + H2O → PbO2 + H2SO4 + 2e-
Negative Plate Reaction
PbO + 2H+ + 2e- → Pb + H2O
PbSO4 + 2H+ + 2e- → Pb + H2SO4
Thus in the charging process, the lead Oxide (PbO2) on the positive plate and the Spongy lead on the negative plate is formed.
Google Source Image
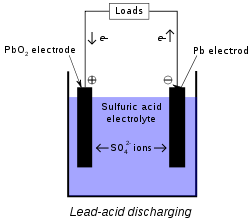
Discharging Process: When the battery is connected to a load in the discharging process, the electric current starts flowing. In this process, hydrogen ion (H+) toward the positive plate and oxygen ion (O-2) move towards the negative plate.
Chemical Reaction is as Follows:-
Positive Plate Reaction
PbO2 + 2H+ + 2e- → PbO + H2O
PbO + H2SO4 → PbSO4 + H2O
Negative Plate Reaction
Pb + O-2 → PbO + 2e-
PbO + H2SO4 → PbSO4 + H2O
The lack of electrons on the positive plate and the excess of electrons on the negative plate. Therefore, the potentiality of the positive plate becomes more than the negative plate potential, whereby the electrons in the circuit start moving from the negative plate to the positive plate. The direction of electric current is the opposite.
Fix Lead Acid battery! Repairs conditions
Continuous use of the battery decreases its efficiency. After some time the battery stops working. this is due to the heat generated by the charging and discharging process. As the temperature increases[3], the water present in the battery is vaporized, the reduction of water inhibits chemical reactions.
We will try to repair the batteries. But we have to keep in mind that the battery is not physically damaged. Lead acid battery works on chemical reactions, the acid used in the reaction is harmful to the skin. Therefore the battery is completely sealed in the Plastic box. It can be repaired without tampering the electrode and internal plate of the battery. To make the battery useful, the lack of water in the chemical reaction must be met. For this, mix a few drops of distilled water inside the battery. And charge the battery on the appropriate volts.
This method of repair is only applicable in the battery of inverter, car and motorcycle. Repairing the battery of flashlight, toys and other devices is a waste of time. PbSO4 made on the plate by the evaporation of water in small batteries becomes so rigid that they can not be changed in Pb or PbO by the charging process. Thus the cell gets damaged.
Reference
- "Gaston Plante" http://www.corrosion-doctors.org/Biographies/PlantelBio.htm
- "Camille Alphonse Faure" https://www.upsbatterycenter.com/blog/camille-alphonse-faure-1840-1898/
- "Battery Temperature" https://batteryuniversity.com/index.php/learn/article/charging_at_high_and_low_temperatures
Thank you for your contribution. Dont forget to link references and sources when applicable!
=======================================================================================
This post was upvoted and resteemed by @Steemgridcoin with the aim of promoting discussions surrounding Gridcoin and science.
This service is free. Please follow @steemgridcoin if you want to support this initiative.
Have a nice day. :)
Warning today car battery's in the US and light trucks are sealed units. It would be very dangerous to play around with them. Acid in car & light trucks battery's is very corrosive and burn flesh! If it gets in someone's eye call 911 ASAP. Also with the EPA you can run into other issues. It is cheaper in the long run to buy a new battery or take it to a mechanic or car dealer.
Here is a link to Material Safety Data Sheet's - https://www.sbsbattery.com/PDFs/MSDS-Battery-Wet-Acid.pdf Link came from Bing search.
Very Nice. Acid is dangerous. Playing with them can harm us.