"Water membranes"
The cell is more negatively charged at −70 mV compared to the extracellular fluid because it has more structured water: water in the form of an hydroxide-rich “gel”, with a molecular structure analogous to ice without the hydrogen ions to bind the lattice sheets vertically. The cell has more structured water, because water is structured by contact with non-water components of the cell, such as macromolecules like actin and the cytoskeleton, down to atom-sized solutes like ions.
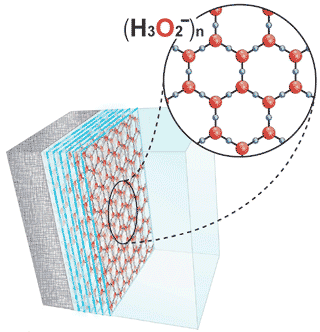
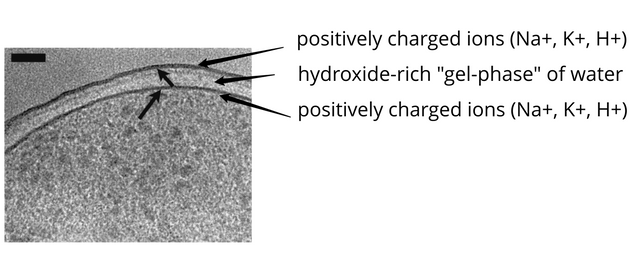
The negatively charged “shell” or “membranes” of water around proteins in the cell, scale up into the negatively charged membrane around vesicles such as secretory granules storing hormones like insulin or acetylcholine neurotransmitters at the axon terminal in a neuromuscular junction, and further into the negatively charged membrane that surrounds the cell itself, bounded by a sheet of positively charged ions such as sodium, potassium, and hydrogen, giving the plasma membrane the contrast-rich appearance seen in electron photographs, with a clear region in the center of solute-free gel-phase water.
These membranes dissolve with detergent in the same way it breaks surface tension, itself a gel-phase water membrane. They are also the mechanism that drives osmosis, solutes decrease the thickness of "water membranes" in the same way they the lower freezing point, both colligative properties, i.e. they depend upon the concentration of solute molecules or ions, but not upon the identity of the solute. Less hydroxide-rich "gel-phase" water in the hyperosmotic fluid means less of its corresponding cation hydronium, compared to in the hypoosmotic fluid. It is this membrane potential that drives osmosis, an electrical gradient "pushes" (Greek ōsmos, "push") hydronium ions through the membrane.