Luminescence - when nature shines

Welcome to my latest article dear readers. In the sixth episode of Myth or Fact we investigated the question of whether radioactive contaminated waste glows green or not. The results were that radioactive material does not glow. But there are indeed materials that can emit visible light. Dials used to contain radium and a phosphor. This combination results in a bright green glow, however it’s not the radium that glows, it’s the phosphor. In this article we will learn what luminescence is and how it works. With many colorful pictures and detailed explanation we will get to the bottom of the matter.
Fluorescence and phosphorescence
Luminescence is the emission of visible light of an object under the influence of energy. Depending of the kind of energy there are different types of luminescence.
- Chemiluminescence – results by a chemical reaction
- Mechanoluminescence – results by applying mechanical force on the object
- Electroluminescent – made by electric current
- Photoluminescence – made by absorbing photons
- Radioluminescence – results by ionizing radiation
- etc.
Regardless of the type of acting force the physical process behind the luminescence is the same. An electron is excited from a lower state of energy to a higher level. The higher energy level is unstable for the electron so that it moves back to its unexcited ground state. The surplus of energy is emitted in form of photons.
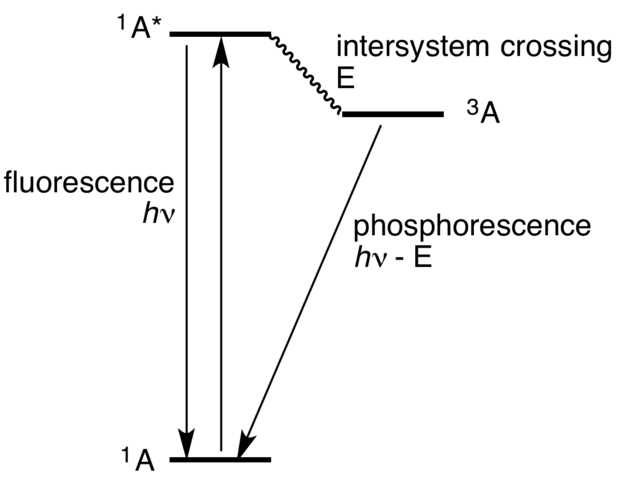
Fig. 1 Jablosnki diagram. Credits
The illustration above explains how fluorescence and phosphorescence are accomplished. The first step is that an electron absorbs a photon. The arrow from A to A' shows the absorption. Only light with a certain wavelength can excite an electron. With the right wavelength the electron is excited from the ground state (A) to the state of higher energy (A'). At this point there are several ways how to “deal” with the surplus of energy. The first possibility is the fluorescence. The electron falls back to its ground states and emits the energy difference E=hv between A and A'. The second possibility is the intersystem crossing. The spin multiplicity changes there from a singlet to a triplet state. Simply said this gives the electron the possibly to enter a third spin state. The transition from A' to A3 is nonradiative. From this excited state the electron emits a photon with the energy E= hv-E(energy difference between A' and A3). The energy of emission is lower than the energy of absorption. The difference is also known as Stokes-shift. Besides these radiative transitions molecules can radiate heat to dismiss energy. After exciting the electron with energy it emits photons. The lifetime of a triplet state is much higher than a singlet state. Because of this fact the phosphorescence can last minutes while fluorescence lasts only up to 10^(-8) seconds. Does a light bulb use luminescence? No it doesn’t. Light bulbs work with heating the wire filament inside. The processes of emitting photons with heat is called incandescence, luminescence works on a cold level.
Practical application
Luminescence has a wide range of application humans and other organisms use. We will have a look at few examples of fluorescence, phosphorescence and bioluminescence. As mentioned a certain wavelength is needed to experience fluorescence. One security feature of the euro bills is the use of fluorescent pigments. Under UV-light these pigments shine brightly in different colors.

Fig. 2 50€ under UV-light. Credits
Due to the higher lifetime of phosphorescence they are often used in security signs. In case of a blackout the signs show the path to the rescue zone. Some of us might remember these little star stickers we had. At day they charge and at night they glow.

Fig. 3 Exit sign glowing. Credits
Another fascinating way of emitting light is the bioluminescence. There are few living organisms that use luciferin. The enzyme luciferase makes luciferin react with oxygen to oxyluciferin, an excited molecule. This molecule then emits photons.

Fig. 4 Firefly glowing because of luciferin. Credits
I bet E.T uses the same principle!
Thanks for reading and until next time,
Tim
great article !
Thanks solider!
Nice one!
Thanks for the great article. I'm looking forward to the time when city streets will be illuminated with live genetically modified flowers and trees.
I would be happy with more trees in general :) but yeah, slick
Opened my eyes to see the light in more depth. Thanks.
Upvoted and followed already.
Thanks for this :) resteemed!
Thank you very much goldmatters
A cool piece!
Thanks puffin! I enjoy your newspaper
But as it is a timsaid piece, these words seem redundant ;)
Then I will write these words on your next post
Very good article, I learned some interesting facts about glooming in the nature, thx for explaining ;-)
Hey Pery, thanks for your feedback. There are so many fascinating things we have to see
I like the glowing firefly the most; I always wanted to know what the effect is. Thx
Nice informative article!
I love your ideas @timsaid in "Myth or Fact" and would like to research and post articles from this niche too. Would you be so kind and give me a hint where you get the ideas for this category? Thx for your help (if possible)!!
Mostly what I heard from other people. Rumours :)
Thx that sounds easy; then you do a research and some sort of mixing text parts or do you a complete rescribe?
This post has been linked to from another place on Steem.
Learn more about linkback bot v0.4. Upvote if you want the bot to continue posting linkbacks for your posts. Flag if otherwise.
Built by @ontofractal