Is sodium and potassium the zinc and copper of a galvanic cell?
Potassium is adsorbed to the cell gel protein matrix, analogous to Zinc plated onto an anode in reduced form. Activation of the cell increases the oxidation state of the potassium ions in the anode (resting state cell), freeing an electron that enters the external conductor. This electron will reduce sodium that plates onto the cathode (active state cell). The cell will then recharge, and return charge separation in the resting state (adsorbed potassium. )
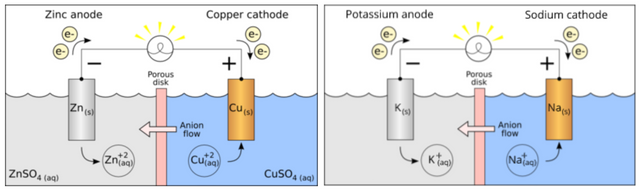
Full redox reaction: Na(s) + K+(aq) --> Na+(aq) + K(s)
To the question in your title, my Magic 8-Ball says:
Hi! I'm a bot, and this answer was posted automatically. Check this post out for more information.