Diffusion in Porous Media – A world of applications – Part 2
Hello, dear friends of Steemit.
In the previous post, I mentioned that I have a degree in Physics and currently I work as a professor in the Faculty of Sciences of the University of Zulia in Venezuela. I also work in the investigation of transport phenomena such as the diffusion of matter and thermal conduction in porous media. Currently, I am tutor of three research projects on the diffusivity or constant diffusion and simulation of the diffusion in porous media in two dimensions, being my research students @dojeda, @taborda-charrouf and @joseg.
The diffusion is a physical process of great importance in physical, chemical and biological systems.
There are two approaches to study the notion and the phenomenon of diffusion: the phenomenological approach using the so-called diffusion laws of Fick and their mathematical consequences, and the approach statistical physicist, which considers the random path of the diffusing particles propelled by its thermal energy.

In porous media, the diffusion is one of the decisive processes in the transfer of matter, for example, the passage of nutrients from the blood to the interior of the cells through in the cell membranes occurs through diffusion.
In general, the diffusion process is described by the so-called Fick Equations of Diffusion, but in porous media, such equations must be modified to include the effects of the geometry of the medium on the movement of the diffusing particles. The procedure for making such modifications I will explain in the following parts of the series.
In the first post, I talked about the porous media, their types and their characteristic properties.
In this second post, I will talk about the diffusion process through the phenomenological approach, their types and the Fick equations of diffusion.
WHAT IS THE DIFFUSION?
The word diffusion comes from the Latin diffusione, which means action of spread, which in turn comes from the Latin diffundere, to spread everywhere, to spread widely, which perfectly describes what happened during the mentioned phenomenon.
Now, formally the diffusion (ordinary) is defined as the net movement of particles due to a concentration gradient, that is, it is the process by which matter (atoms, molecules or other particles) is transported or transferred from a region of high concentration to a region of lower concentration (the concentration is the amount of substance contained in a certain volume).
In the following animated figure, we can observe how the diffusive flow of a group of particles occurs, which can be atoms or molecules of some substance.

Initially, the red particles of the figure, which represent molecules of some substance, are confined to a small region of the container, which means that in that region we have a high concentration of the substance, and in the rest of the container a low concentration of particles (exactly the concentration is zero in the rest of the container). When removing the “barrier” that keeps confined the particles, they tend to move towards the space of lower concentration, occupying the entire container, until the concentration of the red particles is uniform, that is, it is the same throughout the container.
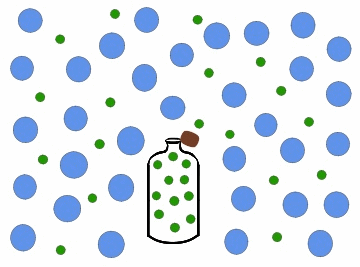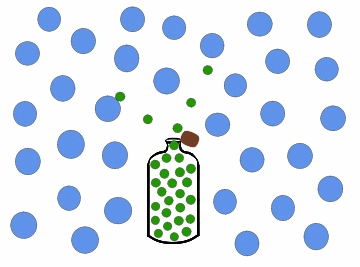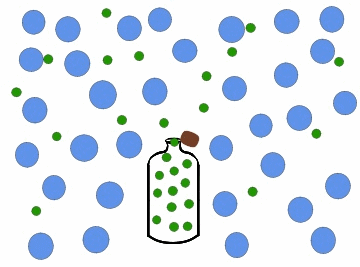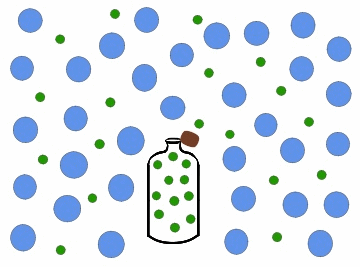
In the previous figure, we can observe one of the most common cases of diffusion in daily life, which occurs every time we open a bottle of cologne. Inside the bottle, the colony concentration is high, and it is zero in the surrounding region, for example, a room. When we open the bottle, the perfume particles move towards the region of least concentration, until they occupy the entire room, so that the colony concentration in it is uniform.
TYPES OF DIFFUSION
The “driving force” or the “cause” of the diffusion can be the concentration, the temperature or the pressure, the most common being concentration. According to the cause, the diffusion can be:
Ordinary Diffusion: When is due to a concentration gradient.
Baric Diffusion: If the diffusive movement is due to a pressure gradient.
Thermal Diffusion: When the diffused substance moves at the mercy of a temperature gradient.
Forced Diffusion: The movement is due to the action of external forces acting on the system, such as a magnetic, electric or gravitational force.
LAWS OF THE DIFFUSION
While, the diffusion has been studied by many scientists, two of the laws (and their respective equations) most used to describe them are due to the German doctor Adolf Fick, with studies not finished in Physics and Mathematics, who published them in 1855 at the age of 26 age. Currently, they are known as Fick's laws.
- 1st Fick's law
Adolf Fick experimentally observed that the diffusive flow is proportional to the concentration gradient, that is, the particles that spread they move from a region of high concentration to one of low concentration.
To clarify the statement given by Fick, it is necessary to define the flow of a substance and the concentration gradient of a substance.
The flow of a substance is the quantity of particles of that substance that cross a surface of unit area in a second, for example, the quantity of molecules that cross a surface of area 1 cm2 in a second . The concentration gradient is the change or variation in concentration per unit length in a given direction.
This experimental or empirical law, for the case of a substance that diffuses in one direction, is expressed mathematically as follows:
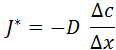
where:
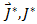
it is the flow of the diffused substance.
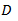
is the proportionality factor, called * diffusion coefficient * or * diffusivity * of the substance in the medium.
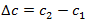
is the variation or difference of concentration of the substance between two points.
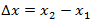
is the distance between two points in the medium.
In the following figure we can observe the flow of a substance through a unitary surface, that is to say, of area equal to a unit.
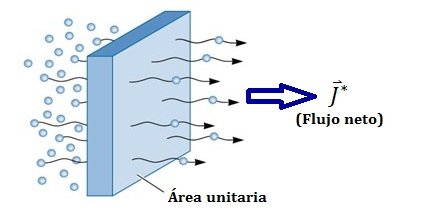
The diffusivity is a measure of the mobility of the particles that diffuse, having dimensions from length by speed (cm2/s).
In the general case of a substance diffusing in all directions, the 1st Fick's Law is expressed mathematically as:
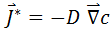
where:
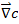
is the gradient of the concentration of the substance.
It is convenient to clarify that, the minus sign in the 1st Fick's Law indicates that the substance diffuses in the direction in which the concentration decreases.
- 2nd Fick's law
Adolf Fick, intuited that the diffusion of a substance was a phenomenon analogous or similar to heat transfer by conduction, and so based on the equation proposed by the French physicist Joseph Fourier to describe heat conduction in 1822, he proposed an equation similar differential for the description in time and space of the diffusion of a substance. This equation is known as 2nd Fick's Law, and it is the most used to explain the phenomenon of diffusion quantitatively.
For the case of diffusion in one direction, for example, in the direction of the axis "x", take the form:
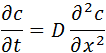
where:
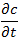
represents the variation of the concentration of the substance over time.
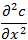
represents the variation of the concentration of the substance in the direction of the axis "x".
In the general case of a substance diffusing in all directions, the 2nd Fick's Law is expressed mathematically as:
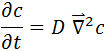
where:
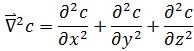
is the Laplacian of the concentration of the substance, and represents the spatial change of the same.
APPLICATIONS OF THE DIFFUSION
The diffusion as a transport mechanism of matter has an extensive presence in nature and a wide range of applications.
Between the biological and medical applications we have the release in the stomach of a drug, the penetration of a drug into patches through the skin, the migration of photons of laser light through the skin, the absorption of nutrient ions by the cellular membrane through specific proteins.
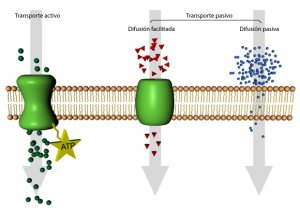
In geophysics, the diffusion has been widely used in erosion studies of slope, cliff erosion, degradation of fault escarpments, removal of the coast, incision of alluvial channels, removal of coastal platforms and delta progradation, among others.
In the industrial field, highlights the surface hardening of steel by modifying the chemical composition of the surface by the diffusion of some chemical element such as carbon.
In the science of materials, we have semiconductor doping by electron diffusion, to increase its electrical conductivity.
In porous media, the difusion involves aspects of experimental observation, theoretical description and technical applications, being the field of study of extensive areas of the science and the technology, among the most important of which can be mentioned the science of soils, hydrology, ecology, oil production and catalytic reactors, becoming an issue of true interdisciplinary importance. For example, the transport of solid pollutants in aquifers, and the absorption of drugs through the skin, are diffusive processes.
I hope you enjoyed the publication about the research in which I work and that the information is your useful, I will gladly clarify your doubts and concerns about the subject. Thanks for your kind reading.
I wait for you in the next installment, where I will talk about the diffusion process through the approach statistical physicist, which considers the random path of the diffusing particles propelled by its thermal energy.
If you are interested, I leave the link of the previous article of the series:
Diffusion in Porous Media – A world of applications – Part 1
Electronic sources:
Fuentes impresas:
Bird, R. B.; Stewart, W. E. y Lightfoot, E. N., 1996. Fenómenos de transporte, Reverté S.A., México D.C., México, pp. 16-3, 22-1.
Bear, J., 1972. Dynamic of Fluid in Porous Media, American Elsevier, New York, EUA.
Torquato, S., 2002. Random Heterogeneous Materials: Microstructure and Macroscopic Properties, Editorial Springer, USA.
Dullien, F. A. L. y Batra, V. K., 1970. Determination of structure of porous media. EN: Flow through porous media, American Chemical Society Publications, Washington D.C., EUA, pp. 12-23.
Welty, J. R.; Wicks, C. E. y Wilson, R. E., 1984. Fundamentos de transferencia de momento, calor y masa, Edit. Limusa, México D.C., México, pp. 533-733.
Youngquist, G. R., 1970. Diffusion and flow of gases in porous media. EN: Flow through porous media, American Chemical Society Publications, Washington D.C., EUA, pp. 57-70.
Hello @hugobohor,
I love your articles. They are very enlightening.
Diffussion is a very important part of our everyday life even though a lot of people don't realize this.
I actually didn't know about the laws of diffusion. I'll re-read this to properly understand the total concept.
Thanks for sharing!
@melanie00
Hello @ melanie00!
I am happy that my articles you like and also have served to you clarify the phenomena of diffusion. You're right, diffusion is a very important process, for example, cell nutrition is given by diffusion
In the next article I will talk about other aspects of diffusion.
Any doubt you have about it, with pleasure, the I will clarify.
Thanks for reading my post!
Oh wow, there are still other aspects?
Seriously looking forward to your next post.
Thank you!
Yes @melanie00, many other aspects, and many other equations, such as Einstein's for the diffusion!
I hope to publish the weekend.
Thanks again!
Congratulations @hugobohor! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPThank you!
Excellent post my friend. Thanks for sharing!. Regards!
Thanks my friend @tsoldovieri.