The Earth Atmosphere: Ozone Layer
 source
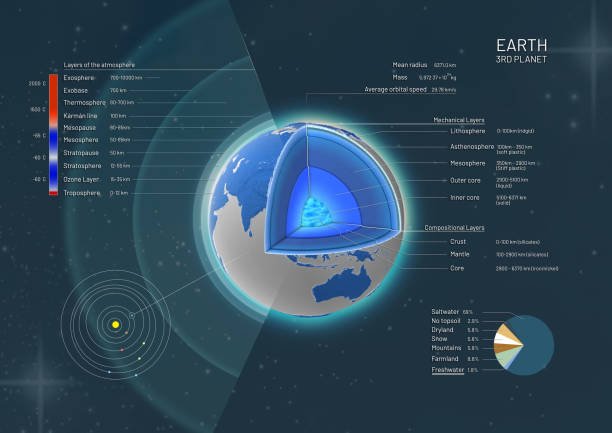
sourceThe earth atmosphere comprises of five main layers and they are;
i. The Troposphere
ii. The Stratosphere
iii. Mesosphere
iv. Thermosphere and
v. Exosphere
The Troposphere is the only layer that living things can breathe in and this is why climbers who climb high mountains go with Oxygen because the air is thinner as you go higher.
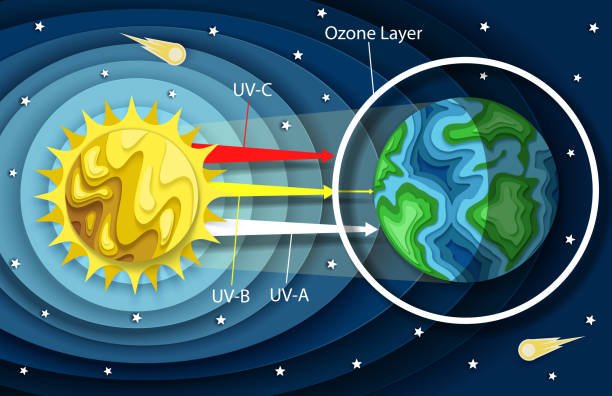
Now the Ozone layer is part of the earth surface in which 90% of the atmosphere ozone is concentrated. The ozone is concentrated in the Stratosphere. While the remaining 10% is concentrated in the upper surface of the Troposphere. In the Stratosphere the Ozone layer absorbs most of the ultraviolet radiation thus leading to increase in temperature. The ozone layer absorbs most of the Sun's harmful radiation and protects living organism on earth. Ozone is thinnest at the equator but it is largest at the poles.
Formation of the Ozone layer and its importance
Ozone layer is formed by a process known as Photolysis where oxygen, molecules absorb ultraviolet photons and goes through a chemical reaction. During this process a single molecule of oxygen is broken down into two atoms. The free oxygen atom combines with an Oxygen molecule to form an Ozone Molecule.These Ozone Molecules absorb ultraviolet radiation to prevent it from reaching the earth's surface.
 source
sourceThe major importance of the Ozone layer is that it absorbs the ultraviolet radiation from the sun thus protecting life on earth.
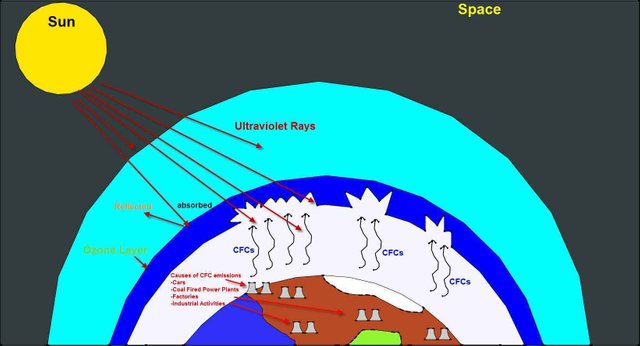
How Chlorofluorocarbon depletes the Ozone layer
In the 1930's CFC was invented by scientists as coolants for refrigerators and production of plastic material. It has become responsible for greenhouse gases emission depleting the ozone layer. CFC is responsible for 80% of the depletion of the ozone layer. When in the Stratosphere chlorofluorocarbon breaks down releasing a chlorine atom because of the ultra violet rays. Since CFC are not easily broken down, they can stay up to sixty years in the atmosphere thus leading to greenhouse warming.
 source
sourceThe effects of destroying the Ozone layer
The destruction of the ozone layer implies that more ultraviolet rays of the sun will enter the earth surface and this can result in the following;
i. Ultraviolet rays can cause cancer, sunburns and cataracts of the eye
ii. Ultraviolet rays negatively surpresses our immune system thus leading to tumor formation and these rays can damage our DNA
iii. Important micro-organisms in the environment will be destroyed.
iv. Complete depletion of the ozone layer will allow direct radiation to earth thus leading to increase in temperature.
Control of Ozone layer depletion
i. Ban the use of products that emits ozone layer depletion like Chlorofluorocarbons. Chlorofluorocarbon causes 80% of the total depletion of the ozone layer. They contribute to greenhouse warming because they are not easily broken down in the atmosphere.
ii. Using products that are ozone friendly.
iii. Chlorofluorocarbon in refrigerators and air conditioners should be recovered and recycled.
iv. Air conditioners in vehicles should be inspected regularly for leads released into the atmosphere.
V. High altitude aircraft flights and rockets should be reduced because they release large amount of green house has to the Earth atmosphere which contributes to the depletion of the ozone layer.
Conclusion
The ozone layer is like a protection that keeps us safe from the harmful radiation of the sun and without the ozone layer life will not exist on earth. The ultraviolet radiation of the sun is very dangerous for all living organism, so by absorbing this ray we are protected from the dangerous radiation of the sun. So it is our duty to protect the ozone layer because without it, it will be doom.
Thank you for contributing to #LearnWithSteem theme (#learnwithsteem , #tutorial, and #lesson). This post has been upvoted by @tucsond using @steemcurator09 account. We encourage you to keep publishing quality and original content in the Steemit ecosystem to earn support for your content.
Regards,
Team #Sevengers
Thank you for providing this interesting and useful information. Ozon's lawyer is very important because it protects everything on our planet. But because of the industrial development and other issues, the Ozon lawyer is damaged, and without its' protection, the consequences may be very bad. Some time ago, I was writing about environmental issues, and the information I found made me a little bit scared, to be honest. Each of us needs to know all that information and do something to protect our planet. Also, on this page https://envrexperts.com/free-essays/essay-about-environmental-and-economy-issues-baldauf, I read a very interesting article related to that theme. And I understand that something like that may not be interesting for some people, but it takes not that much to find and read some related articles, and I recommend everyone to do it and to become more aware.