Why Ammonia and Bleach should not be mixed
Looking at a bottle bleach, a question will come to one’s mind that “why can’t a bleach be mixed with ammonia?” To humans, chlorine gas is a very dangerous gas which is used during World War 1 as a chemical weapon and during World War 2 by Nazi Germany for the same purpose.
Release of toxic vapors called chloramines is the results of reaction of bleach and ammonia in various proportions. Irritation to respiratory system is caused by related compounds called Chloramines. Symptoms such as nausea, watery eyes, breath shortness, chest pain, irritation to nose, eye and throat are the symptoms of exposure to these gases.
What really happen when ammonia is mixed with bleach will be discussed in this article and reason why it is been referred to as dangerous concoction. Firstly, let’s talk about ammonia and bleach in order to know what exactly they are.
Ammonia is a strong and colorless gas in its neutral state with pungent smell. When dissolved in water, it turns to liquid ammonia. The chemical formula of ammonia is NH3. It has one atom of nitrogen and three atoms of hydrogen.The simplest pnictogen hydride is referred to as ammonia. Especially among aquatic creatures, ammonia is a common nitrogenous waste. Nitrogenous vegetable and animal matter is mostly the sources of ammonia and this accounts for why it is found in small quantities in nature.
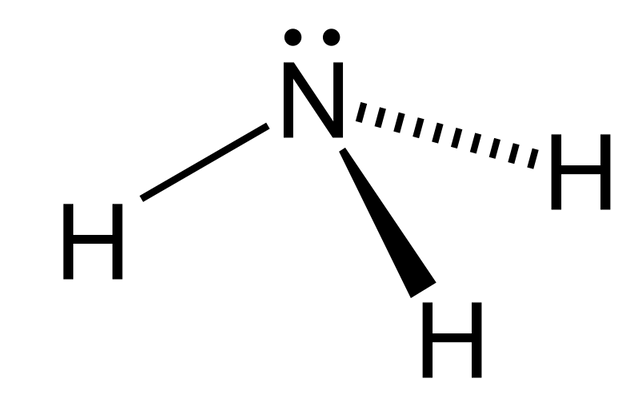
Its density is 0.589 times of air and that makes it lighter than air. Due to the presence of strong hydrogen bond between the molecules of ammonia then it can be liquefied easily.Many pharmaceutical products are manufactured from ammonia and also ammonia significantly contributes to the terrestrial organism’s nutritional needs by acting as fertilizer and food precursor.Besides, synthesis of many household cleaning products required the use of ammonia.
Chemicals that are used in the manufacture of household cleaning product are collectively referred to as bleach. It is a chemical that helps in stain removal and cloth whitening. In the bathroom and kitchen, it is mostly used as disinfectant. In environment where sterile conditions are needed such as bacteria growth control in swimming pools. Bleaches are used for sterile purposes because most environments possess potent bacterial characteristics.
Although few different chemicals combination make up what is called beaches. Most commonly used bleaches have chlorine as the basis of the chemical. For example, bleaching powder has calcium hypochlorite has its active compound and the simply called bleach is referred to as sodium hypochlorite.
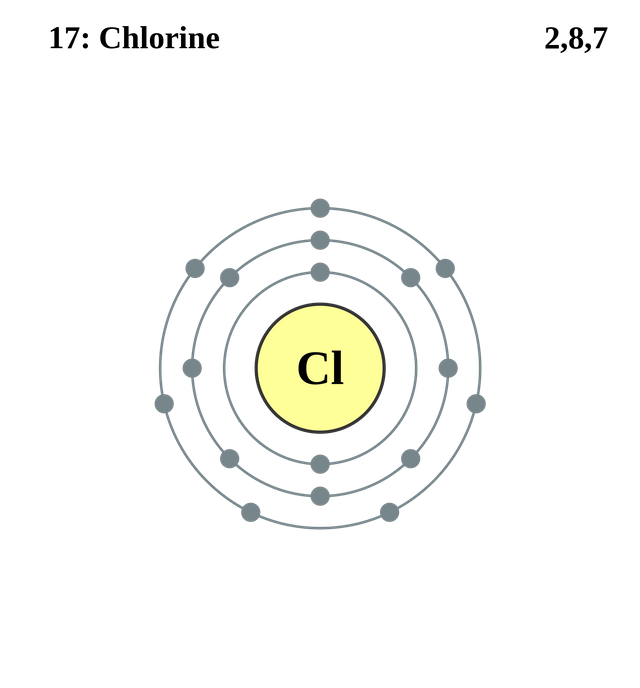
From the reaction above, chlorine gas is released from the sodium hypochlorite. The gas released if inhaled is very harmful and can deteriorate one’s health. Chemical properties of chlorine, number of chemical bond chlorine can form and valence of chlorine has to be understood in other to understand chlorine gas effects on the body. Chlorine belongs to group seven on the periodic table so; they are almost unreactive because the electrons on its outer shell remain one to form an octet.
Octet rule states that outer electron shell must be filled by all elements until they have eight electrons. An element is stable when the electrons on its outer shell are eight. Therefore as a result of closeness of outer shell of chlorine to having eight electrons, it rips other atoms apart as it is desperate to get that last one electron. When chlorine gas is inhaled, this is what happens to your respiratory system.
The gas causes massive cellular damage when it enters into ones nasal passage, lungs and trachea. Obviously, exposure to chlorine gas might not kill immediately but can render ones lung damaged permanently and later result into a death that is painful.
These chemicals can be mixed accidentally in two ways. Firstly by mixing different cleaning products together and it is a very bad idea. Secondly by disinfecting water that contains organic matter with chlorine bleach.
The following chemicals are produced when ammonia is mixed with bleach;
Hydrochloric acid (HCl), chlorine (Cl), chlorine gas (Cl2), chloramine (NH2Cl), hydrazine (N2H4), sodium chloride (NaCl), water (H2O) .
Note: except water and salt, all other chemicals are toxic
Reactions involved in the mixing of bleach and ammonia
The first stage is the formation of hydrochloric acid from the decomposition of bleach
NaOCl → NaOH + HOCl
HOCl → HCl + O
Second stage is the formation of chloramine by reacting ammonia with chlorine gas. The chloramine is released as vapor.
2HCl + NaOCl → NaCl +Cl2 + H2O
2NH3 + Cl2 → 2NH2Cl (Chloramine)
Potentially explosive and toxic liquid hydrazine can be formed if the ammonia is excessively present (depending on the mixture, it may be in excess or may not). Hydrazine is still toxic even if it seems not to explode. Also, hot toxic liquid can be boiled and sprayed by hydrazine
2NH3 + NaOCl → N2H4 + NaCl + H2O
Note: most of the chemicals mentioned in this article are very dangerous chemicals and either consciously or unconsciously these chemicals should not be created because it could result into death or permanent injury both physically and internally.
Precautions to take in case of accidental mixing of bleach and ammonia
In case of accidental exposure to vapor from mixing ammonia and bleach, quickly excuse yourself from the environment to where you can find fresh air and immediately look for emergency medical attention. The biggest danger arises from inhalation of the gases. Mucus membrane and eye can also be attacked by the fumes from mixing ammonia and bleach.
Take the following precautions if exposed to vapors from mixing of bleach and ammonia;
Move away from the environment where the mixed chemicals are. If you are engulfed by the fumes, you might not be able to make a call.
If the situation is bad than you expect, poison control unit should be called for cleaning up of the chemicals and to advice the exposed person on how to handle the after effects of the exposure.
The person that mixed bleach and ammonia should be looked for because there are chances that he or she might be unconscious. The person should be removed to outdoor where there is fresh air if found. Then look for emergency medical attention immediately.
Before returning to the environment where the liquid is disposed, ventilate the area thoroughly. The mistake is likely to be made in the kitchen or bathroom. In order not to hurt yourself, leave the environment and make sure you seek instructions from poison control unit. Give the vapors time to dissipate, pour plenty water to where the chemical is for proper dilution, put on gloves and then do the clean up.
Check out the video below for more understanding about this topic
Image Credits
1
2
3
4
All images are from free sources
References
Hello @sheglow. You did a good job explaining why bleach and ammonia should not be mixed. However, there are two things that I would suggest you do for your future article to enhance readability:
This sentences can be made into point form so that it would appear neat.
It would be better if you write it like this:
That would be nice to read don't you think? This is only my opinion. Good luck and keep on STEM-ing.
Thanks bro. All your corrections are noticed and will be worked on
No problem. Glad I could help.
Well written but what in the world could lead me into wanting to mix ammonia and bleach? Ammonia is not a household product so the chances of mixing the two is rare. I am not a chemistry specialist, perhaps there could be a reason for ammonia and bleach to be mixed in the lab.
Is the post all about precautions to observe in the lab or I'm missing something?
This post can serve as precaution in the Laboratory as well as in the house. Do you know that there are some household cleaning products that contain ammonia?. The only way to differentiate the one that contains bleach and the one that contains ammonia is to look at their label so that you won't go and mix them together. Besides, urine contains ammonia and if it mistakenly get in contact with a bleach in the house there will be formation of chloramine gas which is very dangerous for human health as explained above. Thanks for ur comment @gentlesaid
Ok. That's awesome. Emphasis should have been placed on household products anyway. Well done
Thanks for sharing because a lot of people don't know the consequences of mixing bleach and ammonia because mixing it would generate generates toxic chloramine vapor. Which could be dangerous.
You are welcome bro