Aqua regia : The magical gold dissolver
In 1940 during world war two, in Niels bohr institute in demark, there are two Nobel prize medals made of gold sitting in their laboratory. At the point when Hitler led Germany into demark; they could carry these two Nobel Prize medals. So one of the chemists George Charles De Hevesy suggested that the medals be buried but the idea was rejected by Bohr who was afraid that the medal could be found by the Nazis when they dug the yard up.
Therefore, Hevesy prepared aqua regia and put the gold medals in it which dissolved slowly. When the lab was ransacked by the Nazis, they could only see beakers filled with red-orange liquid and could not find any medals.
Here, the magical aqua regia will be discussed extensively. The chemical reaction of how it dissolves gold and it applications will not be left out.

Aqua regia cannot be ordered because it decomposes quickly as a result of reaction between its components, so this makes it to lose it effectiveness quickly. Therefore, it must be prepared fresh before use.Chloroauric acid which serves as an electrolyte in refining highest quality gold is produced by aqua regia. Specific analytical and etching procedures make use of aqua regia. Glassware of organic compounds and metal particles are cleaned in some laboratory by aqua regia.
Traditionally, NMR tubes were being cleaned with chromic acid but aqua regia method is preferred over the use of chromic acids because the spectra cannot be spoilt by any traces of paramagnetic chromium.
While because of high toxicity of chromium and potential for explosion, chromic acid baths are discouraged. Aqua regia itself has been implicated in several explosions due to mishandling as a result of the fact that the chemical is very corrosive.
While there may be variation in local regulation, careful neutralization would have been the best means of aqua regia disposal, before being flushed down the sink. If the dissolved metal has caused any contamination, the solution that is neutralized should be collected for disposal.
Aqua regia doesn’t have its own chemical formula because it is a mixture of two strong acids. However, HNO3 + 3HCl represent what is called aqua regia, this indicates basically that the mixture is made up of hydrochloric acid (HCl) and nitric acid (HNO3).
Solution of aqua regia is mostly a reddish-orange, yellow, liquid that produces fumes and has a composition that changes rapidly. The traditional aqua regia solution is made up of hydrochloric acid and nitric acid in the ratio of three to one (3:1) respectively.
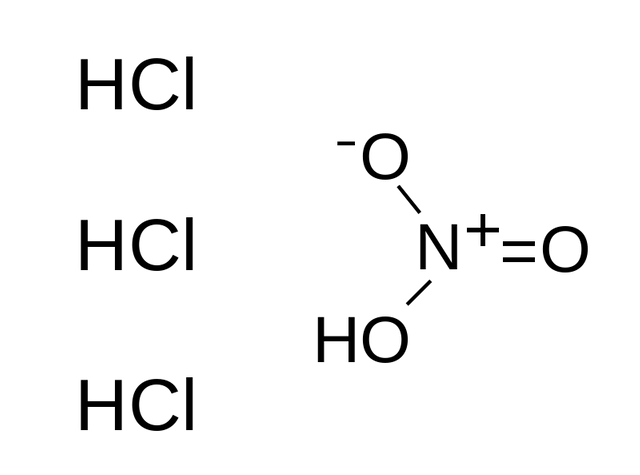
Below is the reaction between concentrated hydrochloric acid and concentrated nitric acid;
HNO3 (aq) + 3 HCl (aq) → NOCl (g) + Cl2 (g) + 2 H2O (l)
Chemical reactions occur upon the mixing of concentrated nitric acid and hydrochloric acid. Nitrosyl chloride and chlorine as evidence by the nature of fuming and yellow characteristics color of aqua regia are the volatile products of these reactions. Aqua regia potency reduces as these volatile products escape from the solution.
Nitrosyl chloride can decompose further into chlorine and nitric oxide. Therefore, the fumes over aqua regia contain nitric acid, in addition to nitrosyl chloride and chlorine
2 NOCl (g) → 2 NO (g) + Cl2 (g)
As a result of the reaction of nitric oxide with atmospheric oxygen, it produces nitrogen dioxide gas
2 NO (g) + O2 (g) → 2 NO2 (g)
Note: Any chemist dealing with these concentrated acids should put on proper protective lab gear just because the acids are very strong acids and with care must the individual handle the apparatus, as the aqua regia is highly corrosive. A very dangerous toxic fume is released by aqua regia which is very harmful if it gets in direct contact with human skin.
Dissolution of Gold by Aqua regia
Gold is being dissolved by aqua regia, although none of the constituent of the acid can do that individually. Each acid performs different function when combined. Nitric acid is an oxidizing agent that is powerful. Through it, undetectable amount of gold is dissolved in order to have gold ions (Au3+).The hydrochloric acid works by providing a ready supply of chloride ions (Cl-).

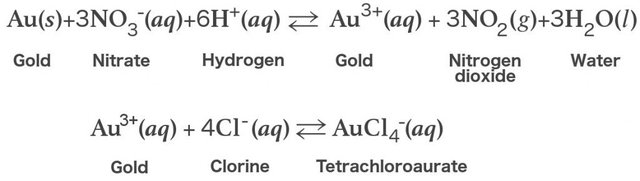
If only gold is present in aqua regia solution, the excess aqua regia may be boiled off in order to prepare tetrachlroauric acid and the residual nitric acid is removed by heating repeatedly with hydrochloric acid. The nitric acid is reduced with this step. The solution may by be reduced selectively by hydrazine, oxalic, sulfur dioxide etc, in case if elemental gold is desired.The appropriated equation for the reaction is;
2 AuCl−4 (aq) + 3 SO2(g) + 6 H2O (l) → 2 Au (s) + 12 H+ (aq) + 3 SO2−4(aq) + 8 Cl− (aq).
Applications of Aqua regia
Since gold can be dissolved completely by aqua regia, then chloroauric acid is produced with this reaction. The chloroauric acid produced is useful in the purification of highest quality gold by using it as an electrolyte in Wohlwill process. Another use of aqua regia is that it is used in the etching of metal and cleaning lab equipment especially organic compound contained glassware.The integral part of gold and platinum extraction and purification processes is aqua regia.
Watch the video below and understand the topic better. Thanks for reading
Images Credits
1
2
3
The Images are from free sources
Reference
- https://en.wikipedia.org/wiki/Aqua_regia
- https://study.com/academy/lesson/what-is-aqua-regia-definition-composition-chemical-name.html
- https://www.scienceabc.com/pure-sciences/aqua-regia-formula-recipe-structure-dissolve-gold-platinum.html
- http://www.recovercomputergold.com/how-to-recover-gold-from-aqua-regia.html
@sheglow have learnt from your post about aqua Regis. Thanks for sharing with us!
You are welcome
Good work. Use text and text to make subscript and superscript respectively for your chemical equations.
Thanks.I usually use it but it seems I have not gotten it.
How did you do that @gentleshaid?