The "Cool" Science: The Reason Moving Air is Cooler Than Still Air
The "Cool" Science: The Reason Moving Air is Cooler Than Still Air
When you and your friends are outdoors on a sunny day, perhaps on the beach, everyone is sweating profusely; you would often wish for the wind or breeze to blow. Why isn't a stationary air good enough to cool your bodies?
[Imgur]Source
This same phenomenon plays out when we stand in front of a fan on a hot day. The breeze from the fan is cool.
The breeze is moving air and is colder than still air. Why is still air not cool? What is so unique about breeze that makes it cool? Let's find out.
Heat Transfer
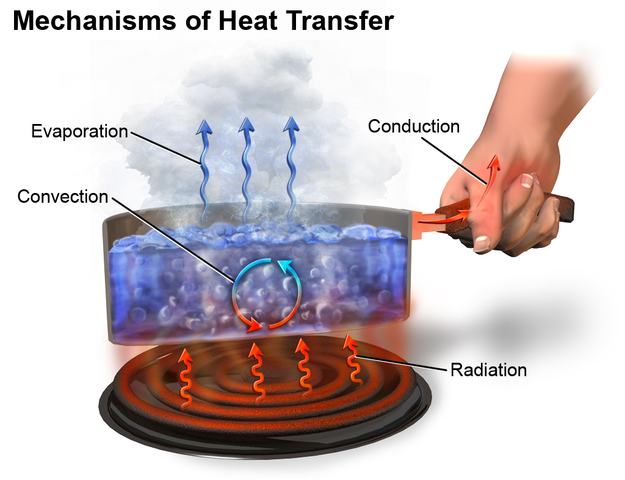
[Wikpedia]Heat Transfer
Heat transfer consists of three mechanisms:
- conduction,
- radiation, and
- convection.
Convection is dependent on the air motion which is a significant characteristic of breeze.
The air is a good insulator and reduces the efficiency of conduction as a mode of heat transfer.
Thermal radiation is limited as air is transparent in a wide spectrum. The radiation, which is the only heat transfer mechanism in space due to lack of air, only contributes to a minor transfer of heat exchange.
The human hairs also tend to resist the small draft and minor convective air flows.
The skin warms any little still air that comes in contact with it if it did not move fast enough to be replaced by colder air. This lack of convection or moving air reduces the conduction of heat away from the skin.
A major player in the game of cooling is evaporation. When a liquid evaporates, the remaining liquid left behind cools. This behaviour is explained by the phase change that occurs as the liquid changes to a gaseous state.
Phase change is merely the big transition energy required as a material move from one phase of matter to the other. This heat of evaporation of water has a value of about 2260 kilojoules/kilogram (kJ/kg).
The moving air increases the rate of evaporation. Thus moisture on the skin evaporates causing a cooling effect.
Evaporating cooling effect
A room that has an air conditioner running is cool. But an air conditioner is more simply defined as a heat pump or evaporator. What it does is to pump heat from one source to the other. It transfers heat in our homes and offices to the outside via evaporation.
[Image Credits]Source
There is a simple experiment to check on the cooling effects of evaporation. Drop a few drops of methylated spirit on the back of your hand and watch as your hand gets cool.
This simple experiment indicates that methylated spirit, which is a combination of methanol and ethanol evaporates readily. This process of evaporation requires energy as was stated above. It takes this energy as a form of heat from the body.
Water does not have this same degree of effect. That has something to do with its boiling temperature. Alchohol has a lower boiling temperature of 78-degree Celcius when compared to that of water at 100-degree Celcius.
To turn into the gaseous state, a liquid would have to break its hydrogen bond that bonds the molecules together. The more the number of these hydrogen bonds, the harder the molecules are to be changed to the gaseous state.
Therefore weaker molecular bond translates to a lower boiling point with increased ease of evaporation. Methylated spirit evaporates faster than water; the increased heat transfer as it turns to gaseous state provides a more cooling effect on the skin.
Moving air accelerates the process of evaporation.
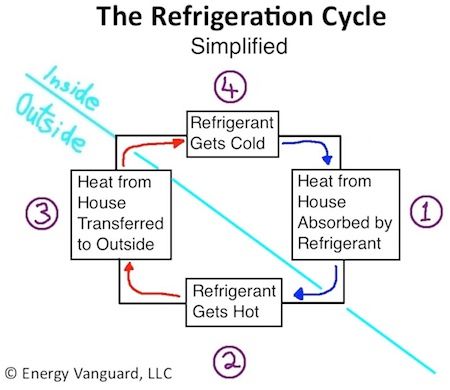
Refrigeration cycle utilises evaporation and follows the heat laws known as thermodynamics. The refrigerant, which is a special fluid that changes from liquid to vapour, pulls heat away from the air at about 23-degrees Celcius (75 degrees Fahrenheit) and pumps it into the atmosphere of above 32°C or 90 °F.
The ac creates a cold area where heat is absorbed by the refrigerator. The heat likes to move from a warmer area to a place that is cooler.
The heat is transferred to a safe area outside. This phenomenon explains why the back of the fridge, freezer and air conditioners are usually hot.
These absorbed heat makes the refrigerants to have a high intensity of heat and thus turns to a vapour. This makes for absorption of heat from the room in the case of air conditioners and makes the room cooler, remember evaporation causes cooling.
The refrigerant cools and condenses (changes from vapour to liquid) in the part called the condenser only to have the cycle repeated.
In other words, the refrigerants absorb heat, evaporates and releases it to the outside air, and gets turned to liquid with the processes repeated.
The Cooling Effect of Moving Air
Air that is moving fast at same temperature produces a faster heat transfer.
Fans give the effect of cooling when it is blowing air towards you. But whether this air would cool or heat you up is dependent on the temperature of the air.
If the air is hotter than you are, the fan would only blow you hotter air. Hence, an air conditioner could just work best in such a situation as it cools by removing heat rather than by blowing it.
To verify this fan's fail at hotter air, touch a metal and plastic object at same room temperature. The metal would appear to be cooler. But that is not so; it merely conducts heat faster away from your hands than the plastic object. In plain words, it cools you down more quickly.
In summary, moving air is cool as it replaces the warm air surrounding your skin with more cooling air hence giving you that cool you so much deserve.
Thanks for reading.
Reference
If you write STEM (Science, Technology, Engineering, and Mathematics) related posts, consider joining #steemSTEM on steemit chat or discord here. If you are from Nigeria, you may want to include the #stemng tag in your post. You can visit this blog by @stemng for more details. You can also check this blog post by @steemstem here and this guidelines here for help on how to be a member of @steemstem.

What I learnt from this article: The refrigerator and the air conditioner have the same principles of mechanisms ....
That is right. Thanks.
Wonderful facts. Moving air and still air. I'm sure those of us that are not science-oriented never knew about this!.. weldone @greenrun.
On the contrary, I think many do.
Hmmm.. interesting!
I never even knew the difference but now i do thanks to you! Good post!
Thanks for reading.
You can never seize to amaze your readers with your good and educative writeups. Now i understand why my room fan blows heaty air most of the sunny afternoon.
You killed it.
Thanks a lot.
You are always *killing me oooo with your content.
This is very insightful sir
Thank me.
@etimbukndem come take a look. Thank you @greenrun Though I knew the reason behind the cooling effect of volatile liquid, I never understood the mechanism that causes the cooling effect of moving air. Now am better informed.
Thanks for dropping by.
That is why we get to feel like tge fan is blowinh hot air. A.c tends to be cool all the time because no matter the situation hot or cold it's job is to take out heat or hotness. I think I get it here.
Thank you for the share.
You nailed it 100% :)
Howdy? Hope you good.
Thank you... am very well.
Wow, I remember the days of high school physics, heat transfer, heat energy, quantum physics,
I regret not listening to those lectures then 😂😂
Good to see you now recognise some of excellent use of education.
Wow.
This is enlightening. I love to learn new stuff.
How then do room heaters work?
An electric heating element, like that found in pressing iron or electric cooker, then the heat is spread in the room by either radiation, convection, or by a fan. Thanks for dropping by.
Thanks for this response and also for upvoting my comment
This reminds me of the principle of cooling by evaporation. The article is a great scientific exposition of the fundamentals of thermodynamics. The work is well researched and presented, I give it an A+ rating. Thanks @greenrun
Thanks for the awesome rating. Do steem on.