THE CHEMISTRY OF AMINO ACIDS AND PROTEINS.
INTRODUCTION
Without enzymes – biomolecule catalysts – the chemical processes of life that takes place in each cell of our bodies would be impossibly slow. When in action, each of these special proteins catalyses its specific chemical reaction with a turnover of thousands – even millions – of molecules per minute.
The suggestion is that enzymes could possibly revolutionize large-scale industrial processes. They are thousands of times faster than inorganic catalysts and most work best at body temperature. Industry uses vast amounts of energy to speed up many reactions, so enzymes could save precious energy. Moreover, they are environmental friendly because it is easy to dispose of them safely.
But there is a major snag. Enzymes lack stability over the wide range of reaction conditions encountered in industry. So the race is on to find ‘extremozymes’ – enzymes that can tolerate higher temperatures and a wider range of other conditions. As their enzymes must be able to withstand high temperatures, organisms that survive in volcanic springs are a promising starting point. By understanding the structure of these enzymes, biochemists can work out ways to synthesize extremozymes that will make industrial reactions more efficient-and greener, too.
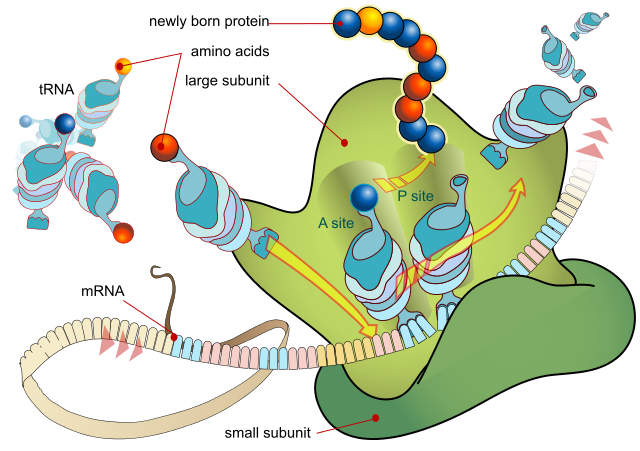
Ribosome, a mRNA and lots of tRNA molecules working together to produce peptides or proteins.
WHY PROTEINS ARE IMPORTANT
Proteins make up about 15 per cent of our body weight. They are the major components of skin, muscle, nails and hair, giving structural support and holding cells together. While these may be the obvious proteins, others are just as essential. These include:
- enzymes that catalyze the chemical reactions going on inside our bodies;
- many protein hormones that act as chemical messengers within and between cells;
- protein antibodies that protect us from disease;
- the protein haemoglobin, which transports oxygen through our arteries.
So, proteins are crucial biological molecules (biomolecules). Yet, despite being so diverse, they all are made up from just 20 different small molecules called amino acids.
NATURALLY OCCURRING AMINO ACIDS
As their name implies, amino acid molecules have two functional groups, an amine group and a carboxylic acid group. All naturally occurring amino acids have the amine group on the second carbon atom of the molecule (next to the carboxylic acid group) This is often called the α-carbon,
2-aminobutanoic acid is an α-amino acid, or a 2-aminocarboxylic acid. The more commonly used term is α-amino acid. In this type of acid, the amine and the carboxylic acid functional groups are both bonded to the same carbon atom. α-amino acids are represented by the general formula shown below.
The side chain R varies considerably, as the figure shows. The composition of the R group confers an individual set of properties to each amino acid, and this, of course, affects the properties of the proteins in which they are found.
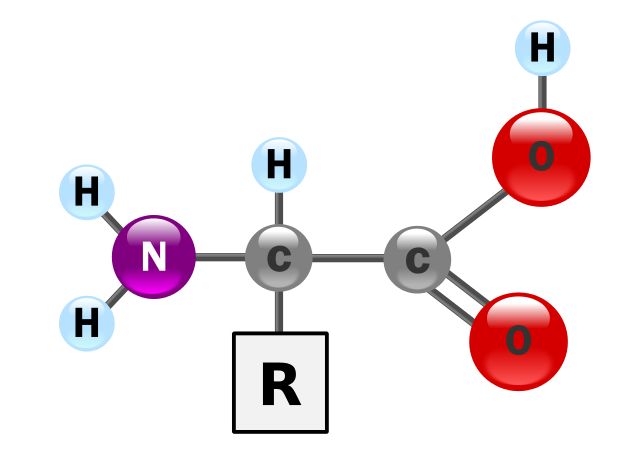
The structure of an alpha amino acid in its un-ionized form. GYassineMrabet, Public Domain
Of the 20 amino acids needed to make up our proteins, eight cannot be synthesized in our bodies. These eight are called essential amino acids and must be part of our diet.
OPTICAL ISOMERISM AND CHIRAL CARBONS
In case we don’t yet know; there are two types of isomerism: structural isomerism and stereoisomerism. The amino acids leucine and isoleucine are structural isomers because they both have the same molecular formula, but different structural formulas. However, with the exception of glycine, all the α-amino acids can exhibit stereoisomerism, which means that their atoms are bonded in the same order but arranged differently in space. One form of stereoisemerism is known as E-Z (cis-trans) isomerism. The other form of isomerism that is shown by these α-amino acids is optical isomerism.
CHIRALITY
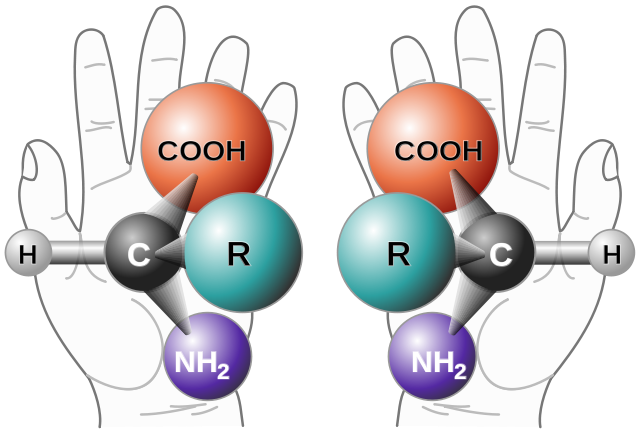
Look at both your hands together with their palms facing you. One is a mirror image of the other. Now put one hand palm up on your table and the other on top of it, also palm up. You will see that you cannot superimpose them – the thumbs stick out in opposite directions. This property is known chirality and exists because hands are not symmetrical. Chiral objects cannot be superimposed on their mirror images – they are non-superimposable. The term chirality is derived from kheir, the Greek word for hand.
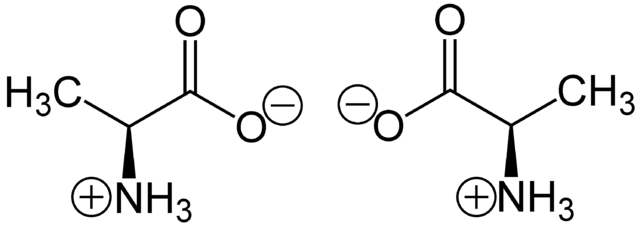
Molecules, too, can be chiral and so have non-superimposable mirror images called enantiomers. In organic compounds, the usual reason for a molecule being chiral is that it has a carbon atom bonded to four different groups. When this occurs, the molecule cannot be symmetrical and the carbon atom is called an asymmetric carbon atom or a chiral carbon.
Take, for example, alanine. It has a chiral carbon because this atom is bonded to four different groups. It is therefore a chiral molecule, having a pair of enantiomers that cannot be superimposed. Looking at glycine, it is observed that its carbon can no longer be asymmetric because it has two identical groups attached (the hydrogen atoms). The glycine molecule and its mirror image can be superimposed, so they cannot be enantiomers of each other.
The enantiomers of chiral molecules have the same chemical properties in ordinary test-tube reactions, which might be expected given that their atoms are bonded in the same order. Although enantiomers have identical chemical properties, they do interact differently with the enantiomers of other chiral molcecules. Hence, some enantiomers do not smell and taste the same, because they interact differently with the chiral taste and smell receptor. However, because their atoms are arranged differently in space, we might expect differences in their physical properties such as melting point, boiling point and solubility. However, these too, are identical, except in one unusual way which gives rise to optical isomerism.
WHAT IS OPTICAL ISOMERISM?
With any pair of enantionmers, one enantiomer rotates plane-polarised light one direction, and the other enantiomer rotates it in the opposite direction. But the angles of rotation are equal. In 1815, Jean-Baptiste Biot, a French physicist, was the first to discover that the crystals of certain substances could rotate a beam of plane-polarised light either to the right (clockwise) or to the left (anticlockwise), looking at the incoming light. At the time, the reason for this rotation was a mystery. It was later discovered that solutions of certain compounds could also exhibit this optical activity.
Two enantiomers of a generic amino acid that are chiral. Wikimedia, Public Domain
Molecules that rotate plane-polarised light to the left (anticlockwise) are said to be laevorotatory. Those that rotate it to the right (clockwise) are called dextrorotatory.
In 1848, the French scientist Louis Pasteur (discussed below) discovered the important connection between the structure of crystals and their optical activity. He was examining salts of tartaric acid when he noticed that samples of the sodium salt included two sorts of crystal, which were mirror images of each other. Pasteur separated the two sorts of crystal and made a solution of each. He then examined both solutions in a polarimeter, an instrument used to measure optical activity. He found that one sort of crystal rotated plane-polarised light clockwise, while the other rotated it anticlockwise. He also found that, when the concentrations were the same, the two angles of rotation were equal, but in opposite directions.
Pasteur proposed that the molecules making up the crystals must also be mirror images of one another. This proved To be an advance of fundamental importance. Less than 20 years later, the tetrahedral model was proposed for those carbon-containing molecules in which each carbon atom has four single bonds. It was then recognized that when a carbon atom is bonded to four different groups, it is asymmetric or chiral.
Many molecules have more than one chiral carbon. For example, the chain form of the glucose molecule has four chiral carbon atoms.
Light and optical activity
Normal light consists of electromagnetic waves that vibrate in all directions perpendicular to the direction of travel. Certain crystals allow light with vibrations in one plane only to pass through them. Such crystals are known as polarisers. The light that emerges from a polariser is called plane-polarised light, or just polarised light. The lenses of Polaroid sunglasses provide a good example of polarisers.
When certain substances are placed between two aligned polarisers through which plane-polarised light is passing, the second polariser stops transmitting light. This is because the substance has rotated the plane of polarisation of the light. The substance is said to be optically active. Rotating the second polariser through a certain angle restores its transmission of light. The angle of rotation caused by a substance is measured in this way, which is the principle of the polarimeter.
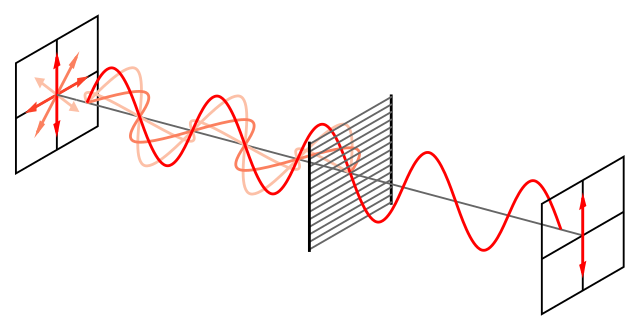 Wire-grid-polarizer. Bob Mellish, CC BY-SA 3.0
Wire-grid-polarizer. Bob Mellish, CC BY-SA 3.0
Louis Pasteur’s great contribution to light and optical activity.
Most people have heard of Louis Pasteur as the inventor of the so-called pasteurisation of milk, heat treatment to destroy the bacteria that are naturally in milk and that would otherwise make it ‘go off’. This process was just one aspect of his revolutionary work on microorganisms and how they cause food spoilage and fermentation.
Among Pasteur’s other achievements was his intense research on silkworm disease, which led to his pioneering germ theory. Putting his theory into practice, he produced vaccines for anthrax and rabies.
Pasteur’s achievements outside scientific medicine were just as significant. For instance, he solved the puzzle of why certain crystals and solutions are able to rotate plane-polarised light. He observed that the sodium ammonium salt of tartaric acid (crystals of tartaric acid are found on wine casks) rotated plane-polarised light to the right, while the sodium ammonium salt of racemic acid (another acid found on wine casks during fermentation) had no effect. Apart from this observation, the salts of racemic acid and tartaric acid appeared to be the same, having identical chemical composition and properties. The shapes of the crystals apparently looked the same. However, under the microscope he noticed right- and left- handed crystals in racemic acid.
Pasteur pressed on, painstakingly sorting the two sorts of crystals with the aid of a microscope and tweezers. He made a solution of each sort and examined both solutions in his polarimeter.
Pasteur’s discovery of right- and left-handed crystals in racemic acid owed much to luck. He was doing his experiments in winter. Above 26°C, racemic acid would not have crystallised out into two different forms. Also, the salt of racemic acid he had chosen to investigate is the only salt whose mirror-image crystals have faces clear enough to allow separation with the aid of a microscope (which would not have been as powerful as today’s instruments). However, as Pasteur himself remarked, ‘Chance favours the prepared mind.’
Racemic acid contains a 50:50 mixture of dextrorotatory and laevorotatory crystals of tartaric acid. Until Pasteur’s separation of racemic acid, the laevorotatory form of tartaric acid was unknown. Any 50:50 mixture of left- and right-handed molecules is called a racemic mixture after the salt of racemic acid that Pasteur separated. In a racemic mixture, the optical activity of one isomer cancels out the optical activity of the other isomer.
Thanks for reading.
REFERENCES.
https://link.springer.com/chapter/10.1007/978-1-59259-709-3_5
https://ghr.nlm.nih.gov/primer/howgeneswork/protein
https://www.webmd.com/men/features/benefits-protein
https://en.wikipedia.org/wiki/Amino_acid
http://www.benjamin-mills.com/chemistry/amino-acids.htm
https://en.wikipedia.org/wiki/Chirality
https://www.chem.purdue.edu/jmol/cchem/opti.html
https://www.britannica.com/science/optical-activity
https://www.encyclopedia.com/science-and-technology/chemistry/chemistry-general/optical-activity
http://chemed.chem.purdue.edu/genchem/topicreview/bp/1organic/chirality.html
https://en.wikibooks.org/wiki/Organic_Chemistry/Chirality/Optical_activity
http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch07/ch7-4.html
https://en.wikibooks.org/wiki/Organic_Chemistry/Chirality/Optical_activity
Congratulations @empressteemah! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPVote for @Steemitboard as a witness to get one more award and increased upvotes!
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @utopian-io.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the curie witness and utopian-io witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having added @steemstem as a beneficiary to your post. This granted you a stronger support from SteemSTEM.
Thanks for having used the steemstem.io app. You got a stronger support!
Hi @empressteemah!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV