Metastasis and cancer #3
Metastasis and cancer #3
I wanted to let you know that I’ve personally commissioned most of the images you will find in this post, I released them to the public domain, thus you are free to use them. I only ask you the courtesy to copy/paste the figure legend citing the artist that drew them and the commissioner (me). If you would like to commission yourself images for science, you can find out how here
 Attribution-ShareAlike CC BY-SA by @pab.ink thanks to @aboutcoolscience and @davinci.art - [Source](https://steemit.com/art/@davinci.art/sketches-cards-pab-ink-n-24-blood-cells)
Attribution-ShareAlike CC BY-SA by @pab.ink thanks to @aboutcoolscience and @davinci.art - [Source](https://steemit.com/art/@davinci.art/sketches-cards-pab-ink-n-24-blood-cells)
 Attribution-ShareAlike CC BY-SA by @pab.ink thanks to @aboutcoolscience and @davinci.art - [Source](https://steemit.com/art/@davinci.art/sketches-cards-pab-ink-n-24-blood-cells)
Attribution-ShareAlike CC BY-SA by @pab.ink thanks to @aboutcoolscience and @davinci.art - [Source](https://steemit.com/art/@davinci.art/sketches-cards-pab-ink-n-24-blood-cells)Previously, I discussed how circulating tumor cells (CTC) are able to exploit our blood cells to survive in circulation and increase their chances of extravasate and form a metastasis. In this post I will try to shed more light on how CTC are able to extravasate and what happens afterwards.
Extravasation
When I say “extravasate” I mean that the CTC have to pass through a wall of endothelial cells. Normally this is no easy task, our body is designed to regulate which cells from the blood get to diffuse in most tissues, however things get easier if the cancer cells start cheating. One trick up to their sleeve is to activate platelets, not only this improves their survival in circulation, but activated platelets secrete ATP which cause the endothelial cells to retract from one another, thus increasing the permeability of the capillary walls (Lambert, Pattabiraman, and Weinberg 2017). Another trick that CTC developed is to induce necrosis in endothelial cells, in other words they simply kill them so to create an opening and extravasate (Strilic et al. 2016).
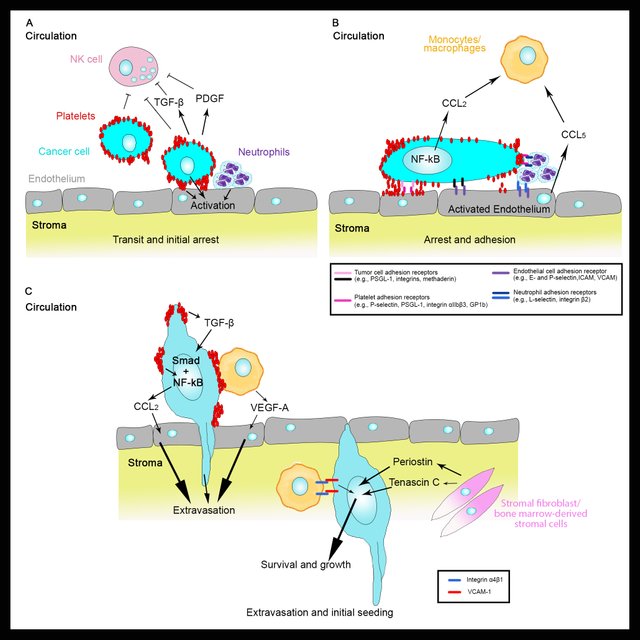 Attribution-ShareAlike CC BY-SA by @elvisxx71 thanks to @aboutcoolscience and @davinci.art - [Source](https://steemit.com/@davinci.art)
Attribution-ShareAlike CC BY-SA by @elvisxx71 thanks to @aboutcoolscience and @davinci.art - [Source](https://steemit.com/@davinci.art)The chances of extravasation are different in different tissues. For example, there is a strong correlation between breast primary cancer and the insurgency of metastasis in the lung. This is probably due to the fact that breast cancer cells when exposed to the TGF-beta secreted by activated platelets, start producing a number of proteins known to disrupt the vascular integrity in lung tissue. Some of these proteins are: angiopoietin-like 4, VEGF, MMPs and ADAM12. As a consequence, breast cancer cells are more likely to extravasate in the lung (Gupta et al. 2007; Reymond, D’Água, and Ridley 2013).
Another trick consists in exploiting monocytes. In particular, cancer cells can secrete CCL2 which can directly increase the permeability of the endothelial cells (Wolf et al. 2012) and also activate inflammatory monocytes that will end up helping the extravasation process of the cancer cells (Qian et al. 2011; Wolf et al. 2012).
So CCL2 may sound like a target for a possible anti-metastatic therapy right? Scientists have tried to probe this approach and unfortunately as soon as the therapy ends they found a dramatic increase in metastasis, this highlights how difficult is to predict the reaction of cancer cells and their actions are truly difficult to counteract (Bonapace et al. 2014).
The good news is that even when cancer cells manage to extravasate, in most cases they die. Someone tried to deliberately inject tumor cells in an animal model and they found that only 0.01% of the cells survived to form a metastasis (take this number with a grain of salt, it’s an estimate) (Chambers, Groom, and MacDonald 2002). The majority of the cells that manage to extravasate either dies or enters a state of dormancy that could last from hours to years (Luzzi et al. 1998).
 Attribution-ShareAlike CC BY-SA by @enmaart thanks to @aboutcoolscience and @davinci.art - [Source](https://steemit.com/@davinci.art)
Attribution-ShareAlike CC BY-SA by @enmaart thanks to @aboutcoolscience and @davinci.art - [Source](https://steemit.com/@davinci.art)So, who awakes the cancer cells?
It’s not clear but we know that the population of the cancer colony is quite heterogeneous, among these cells there is a group of cells that would be the equivalent of stem cells for cancer. These cells are able to wake up their colony or even found new colonies and start the process of metastasis.
References
Bonapace, Laura et al. 2014. “Cessation of CCL2 Inhibition Accelerates Breast Cancer Metastasis by Promoting Angiogenesis.” Nature 515(7525): 130–33. http://www.ncbi.nlm.nih.gov/pubmed/25337873.
Chambers, Ann F, Alan C Groom, and Ian C MacDonald. 2002. “Dissemination and Growth of Cancer Cells in Metastatic Sites.” Nature reviews. Cancer 2(8): 563–72. http://www.ncbi.nlm.nih.gov/pubmed/12154349.
Ghajar, Cyrus M. 2015. “Metastasis Prevention by Targeting the Dormant Niche.” Nature reviews. Cancer 15(4): 238–47. http://www.ncbi.nlm.nih.gov/pubmed/25801619.
Gupta, Gaorav P et al. 2007. “Mediators of Vascular Remodelling Co-Opted for Sequential Steps in Lung Metastasis.” Nature 446(7137): 765–70. http://www.ncbi.nlm.nih.gov/pubmed/17429393.
Lambert, Arthur W, Diwakar R Pattabiraman, and Robert A Weinberg. 2017. “Emerging Biological Principles of Metastasis.” Cell 168(4): 670–91. http://www.ncbi.nlm.nih.gov/pubmed/28187288.
Luzzi, K J et al. 1998. “Multistep Nature of Metastatic Inefficiency: Dormancy of Solitary Cells after Successful Extravasation and Limited Survival of Early Micrometastases.” The American journal of pathology 153(3): 865–73. http://www.ncbi.nlm.nih.gov/pubmed/9736035.
Qian, Bin-Zhi et al. 2011. “CCL2 Recruits Inflammatory Monocytes to Facilitate Breast-Tumour Metastasis.” Nature 475(7355): 222–25. http://www.ncbi.nlm.nih.gov/pubmed/21654748.
Reymond, Nicolas, Bárbara Borda D’Água, and Anne J Ridley. 2013. “Crossing the Endothelial Barrier during Metastasis.” Nature reviews. Cancer 13(12): 858–70. http://www.ncbi.nlm.nih.gov/pubmed/24263189.
Strilic, Boris et al. 2016. “Tumour-Cell-Induced Endothelial Cell Necroptosis via Death Receptor 6 Promotes Metastasis.” Nature 536(7615): 215–18. http://www.ncbi.nlm.nih.gov/pubmed/27487218.
Wolf, Monika Julia et al. 2012. “Endothelial CCR2 Signaling Induced by Colon Carcinoma Cells Enables Extravasation via the JAK2-Stat5 and P38MAPK Pathway.” Cancer cell 22(1): 91–105. http://www.ncbi.nlm.nih.gov/pubmed/22789541.
Communities that support me are:

enlarge
Attribution-ShareAlike CC BY-SA
Reuse this image by copying and pasting this text with it:
Attribution-ShareAlike CC BY-SA by @elvisxx71 thanks to @aboutcoolscience and @davinci.art

IMMAGINE CC0 CREATIVE COMMONS, si ringrazia @mrazura per il logo ITASTEM. Click here and vote for @davinci.witness
I am a technical guy but have little knowledge of biology (I have a fair idea of bio mechanics due to my involvement in robotics).
This is the first time I have come across the term and definition of Extravasation. You have done a really good job of explaining it and thankfully with references (gives me the confidence to read without being apprehensive of the veracity).
Thanks for the cool images as well! Maybe I'll use them one these days....
Would extravasation be the reverse of Epithelial to mesenchymal transition (EMT)? When the circulating tumour cells exit the bloodstream to form a new tumour they undergo Mesenchymal to Epithelial transition (MET) at the new metastatic sites.
I think the cancer cells can undergo EMT even earlier when they are still in the primary tumor. Also this transition is dynamic, they can change back and forth several times, this makes them difficult to target.
Thank you, I'm glad you found the post interesting, feel free to use the images, I had them made for you!
Your figures are awesome! In the second figure you have the monocyte/macrophage also moving with the cancer cell out of the blood stream. I realize there are macrophage-tumour interactions and macrophages can also inhibit apoptotic signals to aid in tumour progression but what is the evidence of these monocytes/macrophages are moving out of the blood stream or lymph with the circulating cancer cells?
its awesome you commissioned all those pics yourself. I have beeb looking for ways to start something of sort, thanks for your recommendation and inspiration.
Hello @aboutcoolscience
Any thanks for images you created and uploaded for public use under public domain license. Researchers and science community will certainly find it very useful.
Though I am a paramedic but will need to do some research to be able to comment objectively on the topic under discourse.
Thank you. And do have a nice week.
@eurogee of @euronation community
Nice and brief information...I really want to know more about cancer especially breast cancer as I have witness many who suffered from this disease. Good works