What is "Hard" Water and why do we need to remove Water Hardness?
"What is "Hard" Water and why do we need to remove Water Hardness?"
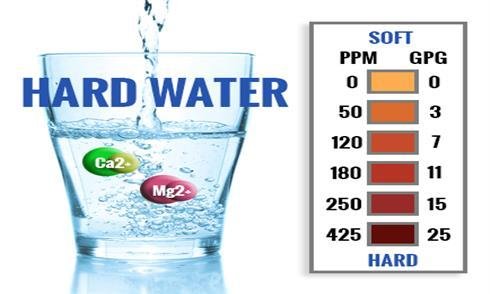
We all use water for many different purposes, either for cleaning, bathing, washing clothes, drinking, etc. Have you ever noticed that your soap seems less ineffective or isn’t forming lather that much? Noticed some deposits in your tap that is hard to clean? Or grayish scum in your bath room? These are all caused by "hard water". Now hard water isn’t really harmful to us, in fact, it has beneficial minerals that is good for us when consumed at the right amount, however using hard water in industrial plants can be a real hassle.
Do you wonder why is it called “hard” water? First of all let’s first define what is water hardness.
Water Hardness is the amount of dissolved calcium and magnesium in the water.
Hardness of water varies according to the quantity of minerals present in your water and it will be considered hard if it has a hardness of 100 mg/L or more.
While, "hard water" is water that contains considerable quantity of dissolved minerals with Calcium and Magnesium ions.
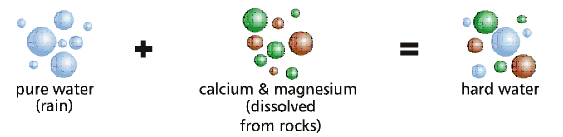
Two types of hardness of water:
Temporary hardness is caused by a combination of calcium ions and bicarbonate ions in the water. It can be removed by boiling the water as this converts the carbonate to insoluble carbonate.
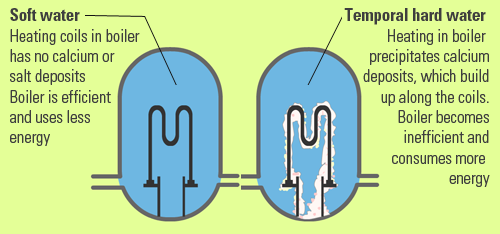
Permanent hardness is hardness that cannot be removed by boiling. It is usually caused by the presence of calcium and magnesium sulfates and/or chlorides in the water.
Measuring Total hardness is just the sum of all hardness.
What is Soft water?
Soft water is treated water in which the only cation (positively charged ion) is sodium, on the other hand, may taste salty and may not be suitable for drinking.
Why do we still need to remove hardness of water if soft water is not suitable for dinking?
Mineral deposits are formed by ionic reactions resulting in the formation of insoluble precipitate. When hard water is heated, Ca2+ ions react with bicarbonate (HCO3-) ions to form calcium carbonate (CaCO3), a precipitate that is insoluble.

This precipitate is also known as scale.
Water and Carbon dioxide will also react to form Carbonic acid, which causes corrosion of iron or steel equipment.
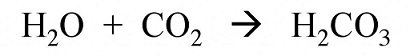
Scale will deposit in Pipes and Tea Kettles
In addition to narrowing and potentially clogging the pipes, scale also prevents efficient heat transfer.


Soap is less effective in hard water because it reacts to form the calcium or magnesium salt of the organic acid of the soap. These salts are insoluble and form grayish soap scum.


Hardness can be removed through Water Softening Processes
Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. Which makes water more compatible with soap, extends the life span of equipment and many other water-based applications.
Some of the methods to remove hardness from water are:
Chemical Process of Boiling Hard Water.
Adding Slaked Lime (Clark's Process)
Adding Washing Soda.
Calgon Process.
Ion Exchange Process.
Using Ion Exchange Resins.
Sources of Hardness of Water
Industrial plants using groundwater as a source of water supply are concerned with water hardness.

During the Hydrologic (Water) Cycle, when water (that is naturally slightly acidic because of carbonic acid) precipitates, some of the water will percolate through the soil and rock, then dissolves small amounts of naturally-occurring minerals like limestone (rich in calcium) and dolomite (rich in magnesium), it carries them into the groundwater supply. Calcium and magnesium, the two principal divalent cations (which means that they have a charge of +2) causes hardness of water.
I HOPE YOU LEARNED SOMETHING
Image Sources: [1], [2], [3], [4], [5], [6], [7], [8], [9], [10]
References:
http://www.eschooltoday.com/water-cycle/what-is-hard-water-and-soft-water.html
http://www.chemistry.wustl.edu/~edudev/LabTutorials/Water/FreshWater/hardness.html
https://en.wikipedia.org/wiki/Water_softening
http://www.tutorvista.com/content/chemistry/chemistry-iii/hydrogen/methods-hardness-water.php
http://www.who.int/water_sanitation_health/dwq/chemicals/hardness.pdf
Great stuff @shairanada. This post is very informative, please continue to do more if this!
Thank you @bayanihan. Will do 😊