The Different of Fusion and Fission in Nuclear Reaction of Chemistry
Chain Reaction
In the 1930s, scientists discovered that some of the core reactions can be initiated and controlled by humans. Scientists usually fire a large isotope with a smaller second isotope (usually a neutron). The collision of these two isotopes can cause the large isotopes to break into two or more elements. In this case, large isotopes undergo nuclear fission (nuclear fission).
This type of reaction also releases enormous amounts of energy. Where did the energy come from? When measurements are made at very high levels of accuracy on all atomic masses and subatomic particles first, then all the atomic and final subatomic mass, then compare them. We will get the result that there is a "lost" mass. The material is "lost" during the core reaction. The loss of this material is referred to as mass reduction or mass defects. This "lost" material turns into energy.
92U235 + 0n1 → 56Ba142 + 36Kr91 + 3 1n0
We can calculate the amount of energy generated from the fission reaction during the core reaction with a very simple equation, developed by Albert Einstein (see: The Story of Scientists, Albert Einstein), E = mc2. In this equation, E is the energy produced; M is a "lost" mass (mass defect); And c is the speed of light (3.00 x 108 m / s). The speed of light squared makes the part of this equation to have a very large number, so that when multiplied by a small amount of mass the result remains a large amount of energy.
At the fission equation of U-235 isotope (see above reaction) a neutron is used. However, the reaction returned to form three neutrons. All three neutrons, when they meet other U-235 isotopes, can start another (fission) breakdown, which will produce more neutrons. This is a domino effect that has long been known to man. In terms of core chemistry, this series of core breaks is called a chain reaction. This chain reaction depends on the number of neutrons released, not the number of neutrons used during the core reaction. When we write the equation of the U-238 isotope (Uranium isotope is more abundant in nature), we only use one neutron and get one neutron as well. Chain reactions can not occur on the U-238 isotope. Only isotopes that can produce excessive neutrons in their solution can experience chain reaction. This type of isotope is said to break. There are only two main isotopes that can be broken down during the core reaction, ie U-235 and Pu-239.
The secret to controlling a chain reaction is by controlling the number of neutrons. When neutrons can be controlled, the energy released can be controlled. That's what scientists do on Nuclear Power Plants (NPPs). In some cases, nuclear power plants are the same as conventional power plants that use fossil fuels. In this type of power plant, fossil fuels (coal, petroleum, natural gas) are burned, and the heat is used to boil water used to make steam. The water vapor is then used to drive a turbine connected to a generator that generates electricity. The real difference between conventional and nuclear power plants is that nuclear power plants generate heat through the chain-breaking reaction of the isotope nuclei.
The advantage of using nuclear power is not necessarily burning fossil fuels (saving fossil fuel sources to produce plastics and medicines) and no combustion products such as CO2, SO2, and others that can contaminate water and air. However, there are still a number of problems related to the use of nuclear power. The first problem is cost. The next problem is the availability of U-235 isotope is very limited. Of all the Uranium contained in nature, only about 0.75 percent is U-235. Most of these are non-divisible U-238 isotopes. The limited amount of nuclear fuel is similar to the limited resources of fossil fuels available in nature. However, the main problem (crucial) use of nuclear power is the level of security of nuclear use and management of nuclear waste. Nuclear reactors must be completely safe and produce no radiation harmful to the health of officers and residents in the area of
a nuclear reactor. In addition, the waste generated must be processed in such a way as to remain safe and harmless to human health.
Nuclear Fusion
Nuclear Fusion
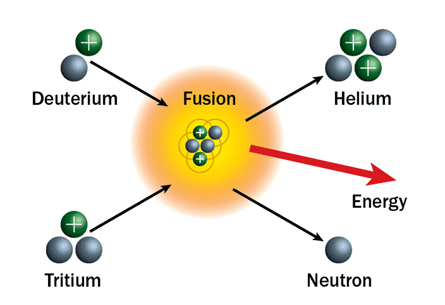
As soon as the breaking (fission) process is found, another process called fusion is found. The fusion reaction is essentially the reverse of the fission reaction. In fission reactions, the heavier nuclei are broken down into smaller nuclei. In contrast, in fusion reactions, the lighter nuclei are combined into heavier nuclei. The fusion process is a reaction that energizes the sun. In the sun, in a series of core reactions, four H-1 isotopes are combined into He-4 by liberating large amounts of energy. On earth, the other two isotopes of hydrogen used in fusion reactions are Deuterium (H-2) and Tritium (H-3). Deuterium is an isotope of hydrogen present in small amounts, but still abundant. Tritium does not occur naturally, but can be easily produced by firing Deuterium with neutrons. The reaction of merging between Deuterium and Tritium is as follows:
1H2+ 1H3 → 2He4+ 0n1
The first core incorporation application was the use of Hydrogen bombs conducted by the military. Hydrogen bombs have a power 1000 times stronger than ordinary atomic bombs.
The purpose of using fusion reactions is to generate abundant amounts of energy. The problem faced now is the difficulty of controlling the fusion reaction. If the energy of this reaction can be controlled and released slowly, it can be used to generate electricity. This will provide an unlimited supply of energy while not producing pollutants that harm the atmosphere.
Radiation Effect
Radiation Effect
Radiation can cause two major effects on the body, which damage cells by heat and ionize as well as break the cell. Radiation generates heat. This heat can damage tissue, just as it does on sunburned skin. In fact, the term radiation burns is commonly used to describe skin and tissue damage due to heat. The main way radiation damages the body of the organism is through cell division and ionization. Radioactive particles and radiation have large kinetic energy. When these particles attack the cells in the body, the particles can break (break) cells or ionize cells, so the cells become ions (electrically charged) by removing an electron. This ionization will weaken the bond and may cause damage, destruction, or DNA mutation in the cell.
Source :
Chain Reaction of Chemistry 1, 2
Support Scientist By Use #science tag or join @steemSTEM
Follow Me @jamhuery



nice informations
thanks for visiting my blog
hai @jamhuery kasaweu lon kiban ile
thank you..beurayek ka votte hehee
@jamhuery got you a $0.94 @minnowbooster upgoat, nice! (Image: pixabay.com)
Want a boost? Click here to read more!
@minnowbooster i followed u from many days but i dont get any upvote from u
meep
https://steemit.com/introduceyourself/@albuluhi/introducetion-new-member-of-team-aceh-steemit-2017728t184325510z I am a new off team aceh.. Vote me.