Really ?? The "Abaca Banana" Can be Made As Kevlar Material ?
Aromatic Polyamide
Aromatic Polyamide
Kevlar or commonly known Aramid in chemistry, it is short name of Aromatic Polyamide. In terms of Chemistry the aramid is an aromatic polyamide which have two monomers namely benzene-1,4-dicarboxylic acid and 1,4-diaminobenzene as well as nylon-6,6 structures molecule.
The combination of two monomers is accompanied by releasing one water molecule. Polyamide is a polymer have units bonded repeater through an amide bond. Physically, Aromatic Polyamide (Kevlar) is a yellow hard fiber, has strong structure, tough, vibration damping, acid resistance, scratch resistant, high form of stability and heat resistance of up to 370°C and at 427°C it will decompose into gas, so it is not flammable.The strength and resistance of kevlar caused by Hydrogen bonding in its molecule (polymer), the bonding of hydrogen can also make kevlar polymer be radial. The kevlar free groups can form hydrogen bonds in outside, so it can absorb the water. The heating resistance of kevlar also comes from of aramid bonding ring, while its strength also affected by the para-phenylene terephthalamide structure.
Single view of para-phenylene terephthalamide
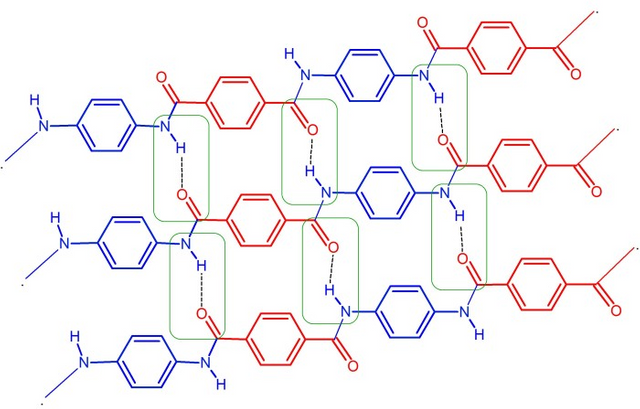 "/>
"/>
Aramid compounds is not easily to react with other compounds and it can be degraded with strong acids, strong bases, or with sodium hypochlorite. But it occur only in high temperatures and take long time, it also sensitive of UV rays that can lead to discoloration from yellow to brown color, the continuous of UV exposure can make it loss of mechanical properties, and Aramid degradation can occur due to UV light with a certain wavelength that has enough energy to break polymer bond and accompanied by oxygen presence.
The Kevlar Forming
The condensation polymer is a process of combining several monomers by reaction of polymer condensation, where in the reaction occurs that releasing of molecules such as H2O, HCl, and CH3OH, this incorporation occurs between reactive groups of the monomers. In condensation polymer reaction, each monomer molecule should have at least two functional groups (bifunctional), there are two types of condensation polymers, natural and synthetic polymer. The natural polymer condensation such as protein polymer, polysaccharide, and deoxyribonucleic acid. Then the synthetic condensation polymers such as the manufacture of nylon polymers, kevlar, and urea. Cross linking occurs in the kevlar polymerization, it is a hydrogen bond that results in a very strong kevlar. The arrangement of monomers in poly p-phenylene terephthalamide:
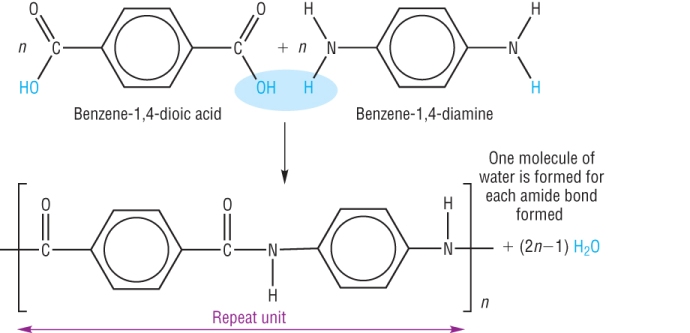
Chemically, kevlar synthesized by polycondensation reaction between terephthaloyl chloride and p phenylenadiamine, this reaction is an interfacial polymerization reaction, ie polymerization requires two non-mixed solvents. The monomers of first solvent will be react in second solvent with other monomers, this polymerization reaction takes fast time. The byproduct of this reaction is hydrochloric acid (HCl). The kevlar is a synthetic pruduk of para-phenylenediamine and terephthalyl chloride (terephthalic acid). The result is an aramid with a benzene ring and an amide group. The polymer sheets randomly assembled when it manufactured, during manufacture process, the material dissolved and spun to orient toward the fiber.
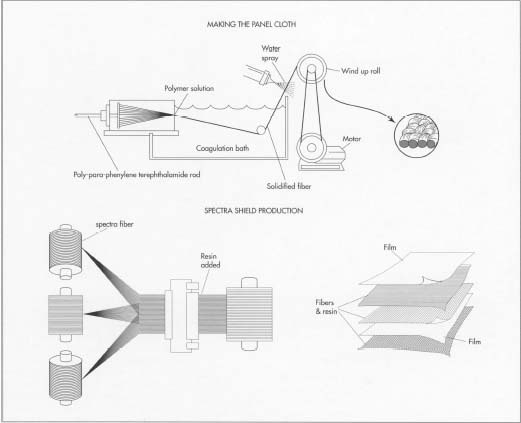 Source :madehow
Source :madehow
Physically, the crystal liquid produced from polymer in rod form then extruded through the spinner to form kevlar yarn, then the fiber passed through the hardened liquid gassy condenser, after sprayed with water, the synthetic fibers are wound onto the roll. Then the kevlar fiber send to the throwsters that twist the yarn to make it suitable for weaving. Yarn woven in simple pattern, only top and bottom patterns of alternate interlacing yarn. Typically, spectra kevlar used in a bulletproof vest is not woven, but the polyethylene polymer filaments are spun into fibers then placed it parallel to each other. The resin (Al2O3) used to coat their sealing fibers together to form a sheet of spectra, two pieces of fabric placed at right angles to bond each other (forming the nonwoven fabric) which is sandwiched between two sheets of polyethylene film.
Kevlar Composite
 Source :alibaba
Source :alibaba
Kevlar was first discovered in the 1970s, kevlar fiber has a high tensile strength, so it is very necessary for composite materials, composite is a combination of two or more materials that have different phases into a new material on a macroscopic scale and have better properties. The main element of the composite is a fiber that bound by interconnected matrix, this fiber material consists of two kinds, long fibers (continuous fiber) and short fiber (whisker). The main element of the composite is a fiber that interconnected matrix bond. This fiber composite material consists of two kinds, long fibers (continuous fiber) and short fiber (short fiber and whisker). The composite particles are a type of composite material whose amplifier consists of particles. By definition the particle is not fiber because it doesn't have a long size, while the composite material, the size of reinforcing material determines the composite ability to withstand the force from outside, where it got longer size, the fiber stronger to withstand the load material from outside.
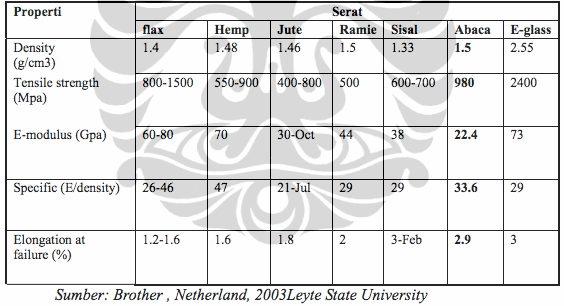
Banana fiber has a high cellulose content of about 60-65%. The resulting fiber is then rolled into a yarn to be sewn into one of the main ingredients of the composite. The working principle of bulletproof vests is to reduce the burst of kinetic energy from bullets as much as possible, by utilizing layers of kevlar to absorb the energy of rate and break it into a wide cross section of bullet vests, so that energy is not to make bullets through bulletproof vests. The absorbing of kevlar energy rate of deformation pressing inward, this inward pressure will continue until the end of composite to withstand the energy rate.
Conclusion
Conclusion
Kevlar is a composite produced from synthetic polymer process, Kevlar has a high pressure resistance and temperature resistant up to 360°C, but the manufacturing process of kevlar is not easy because it requires extreme conditions that use pure sulfuric acid in synthesis process, its strong structure caused cross linking which occurs due to hydrogen bonding from its constituents. Banana fiber contain 65% cellulose and 980 Mpa tensile strength, chemically, it meat minimum requirement to be composite material.
Source :
Support Scientist By Use #science tag or join @steemSTEM
Follow Me @jamhuery

 Source :
Source :
Interesting information.
"Banana fiber contain 65% cellulose and 980 Mpa tensile strength"
Are you saying that weaving together banana skins can be used as a protective body armor?
Its tree fiber :)
thanks
good post thanks
Thanks for reading