Magnetism: Part 5 - The Quantum Mechanics of Spin
In the last post, we saw that the assumptions upon which the Bohr-van Leeuwen theorem were based; namely, classical mechanics, do not 'play ball' when we try to find a description of magnetic materials! We must approach the problem via quantum theory. This post will serve as a rather brief introduction to the quantum mechanics of spin.
Orbital and spin angular momentum
The electronic angular momentum discussed previously is to do with the motion of an electron oribiting around the nucleus, known as the orbital angular momentum, and is dependent upon the electronic state occupied by the electron. With the azimuthal quantum number and magnetic quantum number defined in the usual way, it is the case that the component of the magnetic moment along the z axis is given by
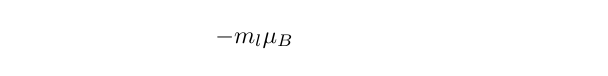
while the magnitude of the total dipole moment given by
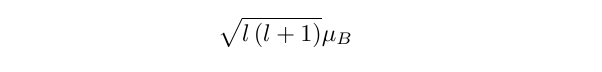
Unfortunately, our life is made yet more difficult due to the fact that an electron has an intrinsic magnetic moment, that is associated with an instrinsic angular momentum, called spin. The name originates from early ideas regarding the precession of electrons around their own axis, but we now know better and that spin angular momentum has very little to do with spin, at all! This can all be a bit confusing - just remember that spin is just another quantum number!
The spin of an electron is characterised by a spin quantum number, s, which for an electron (or any fermion, for that matter) takes the value 1/2. Now, the value of any component of the angular momentum can take one of 2s + 1 values:
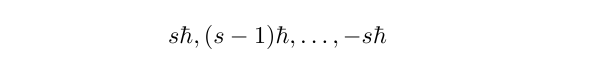
The component of spin angular momentum is written as
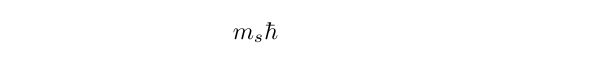
so we can see that for an electron with s = 1/2, there are two possible values, namely
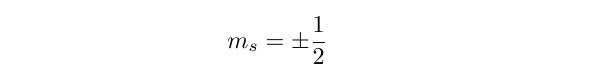
with the so-called up and down components of angular momentum along some axis given (respectively) by
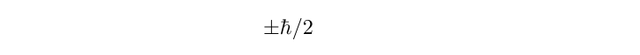
The magnitude of the spin angular momentum for an election is given by
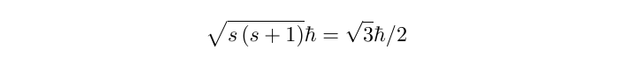
It is the case that the spin angular momentum is associated with a magnetic moment which can have a component along some axis equal to
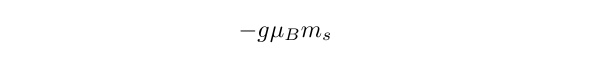
with a magnitude given by
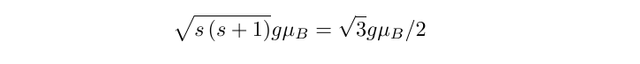
The g-factor in these expressions takes a value of roughly 2, with the energy of an electron in a magnetic field B given by
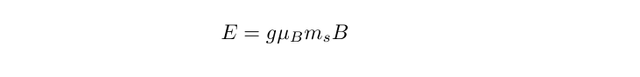
We can see from this equation (the m_s term) that the energy levels of an electron split in the presence of an external magnetic field, in a phenomenon known as Zeeman splitting.
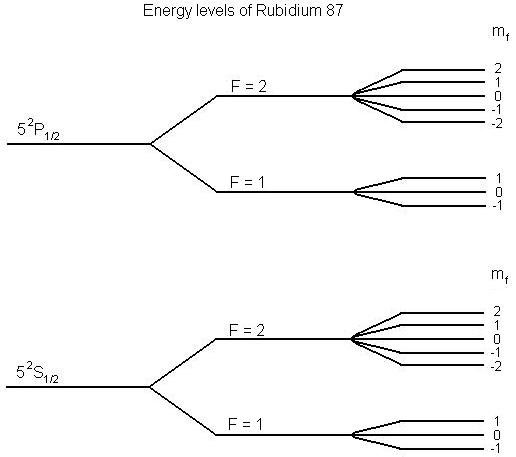
It is, in general, the case for electrons in atoms, that orbital and spin angular momentum are present, which combine. The g-factor can take on different values in real atoms depending upon the relative contributions of spin and orbital angular momenta. The angular momentum of an electron is always an integral or half-integral multiple of hbar, thus it is fairly convinient to drop the hbar factor in these expression, which puts us in the situation where these operators measure the angular momentum in units of hbar.
In the next post, we will introduce the Pauli spin matricies and spinors.