Diamonds Are Not Forever if We Understand its Nature and its Crystal Structure

"Diamonds Are Not Forever if We Understand its Nature and Structure"

One of the most expensive materials on earth is diamond. You might have also heard and believed that diamonds are forever. It is also regarded as one of the rarest stones in the planet. But technically diamonds are not forever and it certainly wouldn’t last forever. Diamonds are also not rare but rather are just expensive. The moment you understand the nature and structure of diamonds you will then realize that diamonds just hyped and overvalued materials. So why are diamonds so expensive?
If we trace back history, we are only told by a certain mining company called the De Beers that diamonds are symbols of engagement and that engagement rings need diamonds. With their money and power, they manipulated the market and have successfully convinced the people especially the Americans that diamonds are to be valued and that diamond engagement rings should be the symbol of marriage! But that was before we understand its nature and nanostructure. So what is a diamond?
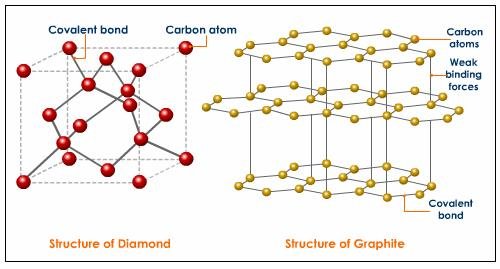
According to Wikipedia, Diamonds are metastable allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. In other words diamonds are just the result of the formation of carbon atoms which are subjected to high temperature and high pressure through time. Diamonds on the other hand are just bonds of carbon atoms which are arranged in a tetrahedral lattice structure. This technically means that diamonds are just composed of carbons.
In fact this was proven way back 1772 when a French scientist Antoine Lavoisier found out that concentrating sunlight with the use of lens into a diamond in an atmosphere of oxygen will produce carbon dioxide as the only product of combustion. An English chemist named Smithson Tennant had verified this by repeating this experiment on 1797 by burning diamond and graphite. Therefore diamond is one of the most common mineral in earth since carbon is the most abundant element in the planet.
Let’s take graphite for example, which is another allotrope of carbon, if we subject graphite at high temperature and at high pressure at the same, the resulting material is probably diamond. Therefore graphite, coal and diamonds are just made out of the same chemical element which is individually arranged to form different crystal structures. However, the properties of each material are of not in great difference. The only special thing about diamond is its hardness due to its strongly bonded carbon atoms which are arranged evenly in all directions.

On 2014, a team of researcher which was led by SLAC scientist has tried to uncover scientific methodology to understand and control the structural transition of a material which is primarily composed of carbon to form another mineral with the same chemical component. In their experiment, they discovered that:
Hydrogen binding initiated a domino effect, with structural changes propagating from the sample's surface through all the carbon layers underneath, turning the initial graphite-like structure of planar carbon sheets into an arrangement of carbon atoms that resembles diamond.
You can read the citation of the research here. Technically, it needs very high pressure to turn graphite in to diamond given the fact that graphite is the most stable form of carbon. But one should not misunderstood diamond to be formed from compressed coal but rather, more likely from sedimentary carbonated rocks and organic carbon deposit.

There’s no doubt that diamonds are the hardest known material so far and are one of the greatest conductors of heat due to its highly compacted structure of atoms. It has a specific gravity of about 3.53 or a density of 3.53 grams per cubic centimeters. Diamond is categorized in the number 10 spot at Mohs scale and is almost 4 times harder than corundum which is number 9 on the same scale. Moreover, due to its formation at high temperature, diamonds have very high melting point.
However, diamonds are not forever, huge amount of force when applied at certain point or spot of a diamond can fracture it. Diamonds are also not worth that much and it is mostly used only as jewelry. Synthetic diamonds and diamond-like materials for industrial purposes are also being manufactured and are being produced in laboratories today. You do not want to invest all your hard earned money for things like that; instead we would like to invest it to corporate investment or asset which is more worth it. Gold on the other hand has the higher value or if you want, you can invest it to cryptocurrency.
THANK YOU FOR TAKING THE TIME TO READ MY ARTICLE
Ceramics Water Filter for Safer and Healthier Drinking Water
Types of Portland Cement and its Individual Use in Construction
An Overview on the Manufacturing Process of Cement Industry
An Overview to Ceramics Engineering & Basic Formulation of Traditional Ceramic Body
References:
http://www.businessinsider.com/heres-why-diamonds-are-so-expensive-2015-9
https://thetechreader.com/tech/diamonds-are-not-forever-they-are-also-one-of-the-most-abundant-stones-on-earth/
http://www.minerals.net/mineral/diamond.aspx
https://en.wikipedia.org/wiki/Diamond
https://www6.slac.stanford.edu/news/2014-03-27-science-bling-turning-graphite-diamond.aspx

The propaganda of De Beers succeeded that the people who are ignorant about it's rarity buys diamond worth even for a million dollar. lol
Well they bought million dollar worth of carbon hahaha!
they could have just bought charcoal if they want carbon so bad hahaha
Yeah, it's much worth the price hahaha!
The main controversy about diamonds is that it has been hoard by companies so that it would be sold cheap. Child labor is a prevailing issue.
Diamonds are cheap anyways as long as you understand its nature.
It is just that the market dictates the price.
Yeah that's it hahaha
kahit love walang forever.... ano na lang pala ang forever?
Blockchain bai? hahaha
Hahaha 😂
Hoi dont be hating on diamonds! Im going to pretend I never read this Chris! 😜
Hahaha buy gold instead of diamonds mommi.
or crypto 😎
Ay wala ba? #ktnxbye!
I didnt know that diamond are not forever but graphene is very promising. This is my case study in our ES 67. Good post.
Basically diamonds are just carbon chain. Graphite is a lot stable than diamond.
One reason why diamond is deemed rare and expensive is because of how nature makes it, including the tedious process of mining it after thousands of years of nature's work on this gem.
Anything derives their value based on what the community accepts its perceived value is.
Take crypto for example. These currencies have value because the community. Throw in signature bags, clothes and shoes on this while at it.
So, one is only as expensive as what we hoomans say it to be.
Hmmm, I see.
Did you know that there exists a diamond dward star?