Fick's Law of Diffusion in Cosmetics: Understanding How Products Penetrate Our Skin (Plus Practical Insights on Skin Care products!)

Have you very wondered why two skin care products with the same active ingredient, will have different effects on skin? Why do cosmetic formulators add so much ingredient in a single product in addition to its active ingredient? Why do some substances remain on the surface of the skin while others penetrate? We can shed light to these questions by understanding how cosmetic products penetrate our skin.

The truth is, most component of the skin products we use every day cannot make it through the outer barrier - it is our skin’s protective mechanism. Let us look closer on how active substances penetrate our skin using Fick’s First Law of Diffusion. Also, with these understanding, let us draw some practical insights regarding skin care products.
Fick's First Law of Diffusion

Meet Fick. This interesting guy is a physicist who later found interest in medicine. His aptitude in both fields of physics and physiology brought about his precious gift to mankind: Fick’s Laws of Diffusion. His First Law of Diffusion, on simple terms, postulates that molecules move from region with high concentration to a region with lower concentration. [1]
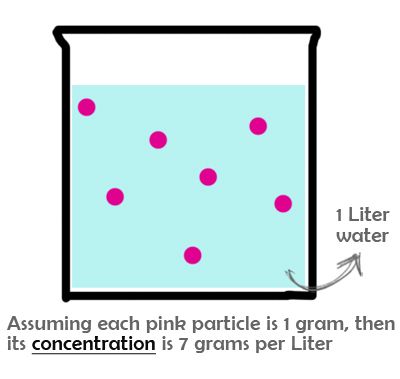
Concentration simply means the abundance of one specific substance divided by the total volume of the mixture.
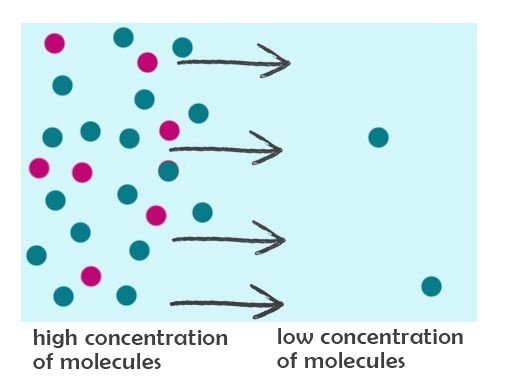
Scenario #1 at time = 0 sec
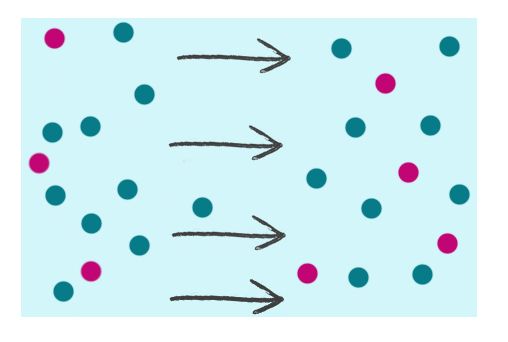
Scenario #1 at time=1 sec
So basically molecules just try to occupy the empty spaces, easy breezy. Looks like just a very normal phenomenon- but Fick observed something more. He found out that the amount of molecules that moves from one point to another per second is directly proportional to the difference in concentration. The higher is the difference of concentration of both region, the greater is the rate of transfer. Now, let's try to have a different scenario at initial time. What if there are many molecules at the right side to begin with?
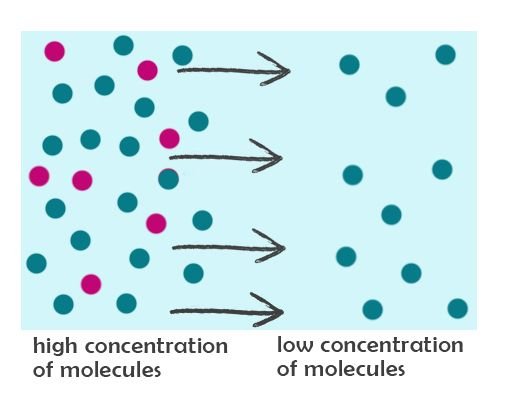
Now, let's again imagine to allow the molecules to travel, for 1 second…..and pause. Let’s take a look at the pink molecules that moved to the right:
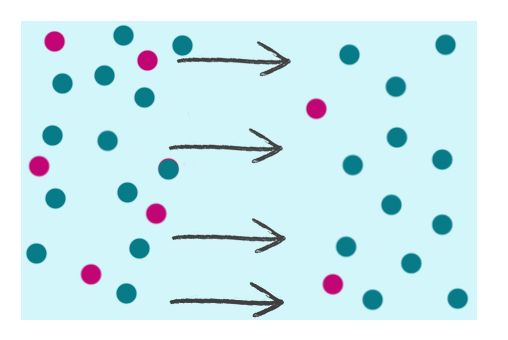
Less pink molecules are transferred to the right side by the end of 1 second because there was initially higher number of molecules at the right. The greater is the difference in concentration, the more molecules are transferred in a given time period. This is a very simplified explanation of Fick’s First Law. This physical phenomenon is modeled in a mathematical equation, now called as Fick’s First Law of Diffusion:
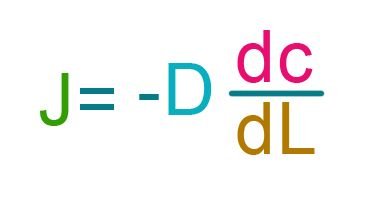
Where:

Where J is the amount of a substance that flows through a unit area, during a time interval (In physics, this referred to as the FLUX). dL is the change in position in space which is also the path length of the substance. dc is the difference in concentration of substance from one point of the path length to the other. [3] The “dc/dL” is a derivative term referring to the difference of concentration relative to its path length. Also, it features a parameter D, the diffusion coefficient. Diffusion coefficient is the inherent property of the molecule and the medium where it is moving which could significant affect the rate of diffusion too.

Diffusion is the net movement of molecules resulting from a random motion. You can imagine this when pouring dye in clear water. If you look in a super magnified microscope, molecules move randomly at all direction, but altogether, they have bulk movement which is visible in the naked eye.
Fick's Law of Diffusion is a very elegant equation used in different fields of Sciences. Though not applicable to all moving matter, it describes many physical phenomena like how gas molecules diffuses through a membrane and how drug is transported through cells.
Cool, So How Do We Relate this to Skin care Cosmetic Products?
Fick’s Law describes a “passive diffusion” where there are no external factors like heat or pressure that may affect the process – much like how substances seep through our skin. To have a better perspective, let us look at equation 2 below which is derived from equation 1. This equation is adjusted [2] so that it will better describe the movement of active substance in cosmetic products through the skin barrier. It also got rid of derivative (because we don’t want to confuse ourselves), assuming that change in concentration is very small as it travels along its path length in the skin:
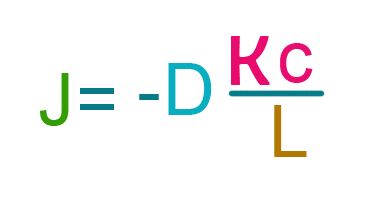
Where:
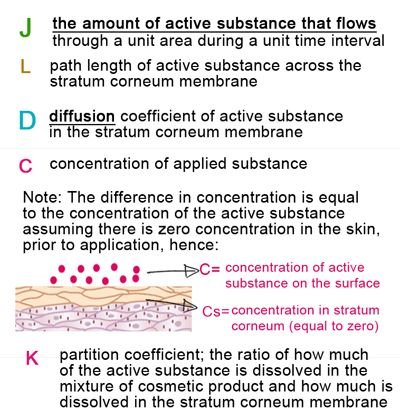
Though the equation above was originally intended to describe passive skin diffusion of drugs applied on skin, we can still apply this to cosmetic skin care products. The parameters are color coded in reference to equation 1. It introduced a new parameter K, the partition coefficient.
Parameter K, the partition coefficient, is simply the value that represents how the active substance is distributed between the stratum corneum membrane and the “vehicle” of the product. As you may notice in product labels, skin care products do not contain 100% active ingredient. Active substance/s are suspended in other ingredients which will serve as its carrier or vehicle.
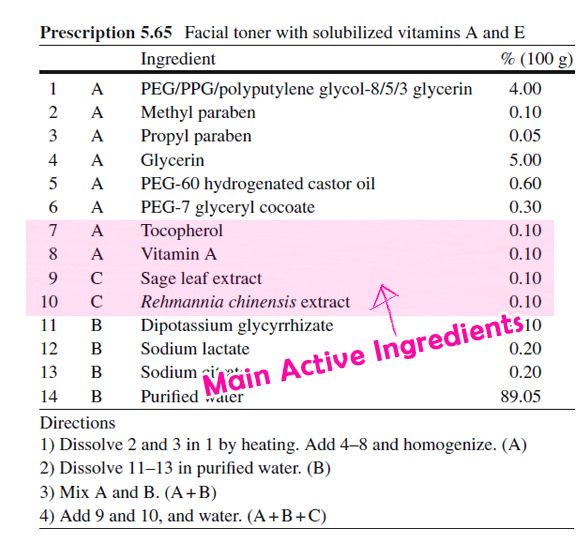
The image above is an example formulation of a facial toner [5]. Active ingredients comprise only 0.4% of the total product formulation. We can imagine the rest of the ingredients as the carrier of the active ingredients.
A Quick Look of The Skin's Anatomy
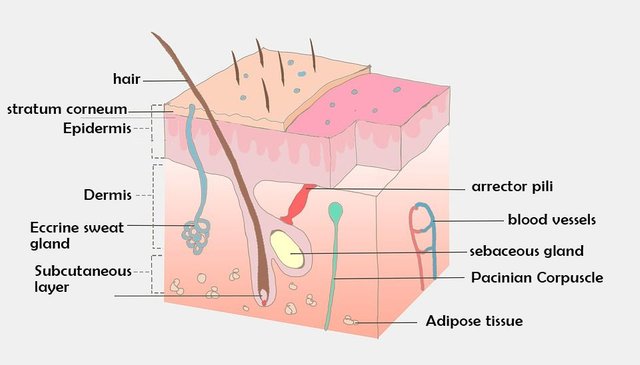
The outermost layer of the skin is called the stratum corneum. This consist mostly of dead skin cells which lies in a bed of lipid (molecules that do not mix with water) matrix. Apparently, this is the main barrier of active substance to penetrate our skin.
So how do skin care products enter the skin? Here’s an illustration of the possible pathways of active compounds in the skin :
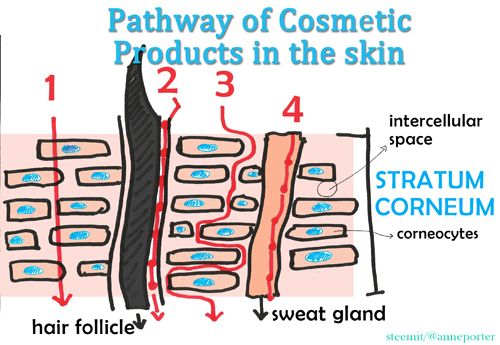
Path #1: Transcellular Route – substance pass through corneocytes (cells in the stratum corneum) and through the lipid matrix
Path #2: Hair Follicle – substance pass through the hair follicles with associate sebaceous glands
Path #3: Intercellular Route - Substance pass in between corneocytes, via the lipid matrix
Path #4: Sweat glands – substance pass through the sweat glands
The skin appendages ( hair follicles and sweat glands ) consist only about 0.1% of the total skin surface area, hence, the principal pathway of substance is through transcellular or Intercellular route. This pathways will be the basis for Fick’s Law of Diffusion. [2,4]
Cosmetic Insight #1: Skin pores, which are the opening of hair follicles and sweat glands, comprise only about 0.1% of the total skin surface. Hence, the “beauty advise” to wash face with warm water to “open pores” and improve product penetration will actually make very little or no difference at all. Most of product penetration is via transepidermal (Path #1 and Path#3) route.
…Also, pores don’t open and close with varying temperatures.
A Closer Look of Fick's First Law of Diffusion : Significance of each Parameter
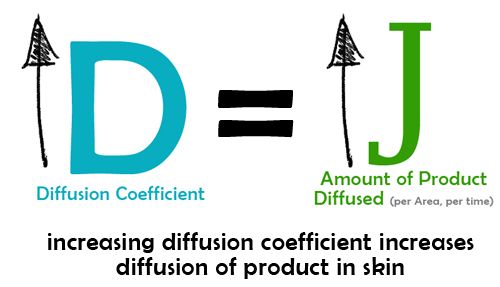
1. Diffusion Coefficient

Diffusion coefficient is a parameter that is influenced by the inherent characteristic of the active substance and the medium where it is diffusing. If we increase the diffusion coefficient, the better is the diffusion rate. Diffusion coefficient can be increased by decreasing the resistance of the stratum corneum.

Penetration enhancers like fatty acids decrease the viscosity of lipid in the intercellular space (see path # 3 -intercellular route). When viscosity of lipids in path 3 is decreased, there is less resistance to flow and active substances can easily take this route. [5]
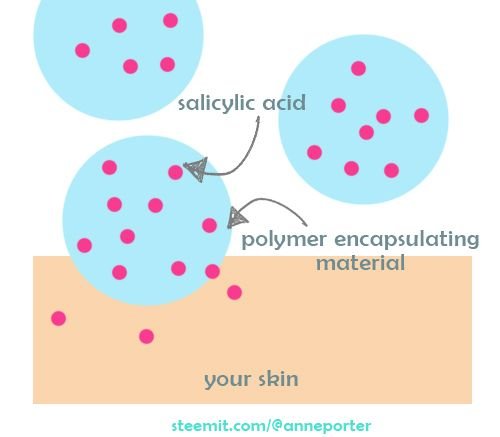
Cosmetic Insight #2: An increase of diffusion coefficient is not always desired. Salicylic acid, for example, can be irritating to the skin when absorbed in high amounts in a short period of time. Encapsulation of salicylic acid in polymers is employed in some cosmetic products for a slow-release formulation. This lessens the risk of irritation. Encapsulating salicylic acid forms a larger spherical structure[9] , which decreases the diffusion coefficient of salicylic acid.

Cosmetic Insight #3: Decreasing the molecular size of active substance can also increase the diffusion coefficient. The smaller is the molecule, the faster it can diffuse through the startum corneum.
Glycolic acid is a common ingredient in toners with exfoliating properties. It is also among the most researched alpha-hydroxy acid for chemical exfoliation. Its efficacy and fast exfoliating properties can be attributed to its low molecular weight, compared to other alpha-hydroxy acids

Cosmetic Insight #4: Collagen, is a structural protein that keeps our skin from sagging. Breakdown of collagen will result to appearance of wrinkles. Most collagen containing products in the market claim to have anti-aging effects. Do these really work?
As a general rule, molecular weight of 500 Daltons will penetrate the skin better compared to substances with higher molecular weight [2]. Collagen molecules vary significantly in molecular size and can reach up to 64,000 Daltons [8]. High molecular weight of collagen molecules significantly decreases its diffusion coefficient and will have very poor diffusivity in the skin. Most of the collagen in these products will just sit on the surface of the skin.
Remember:
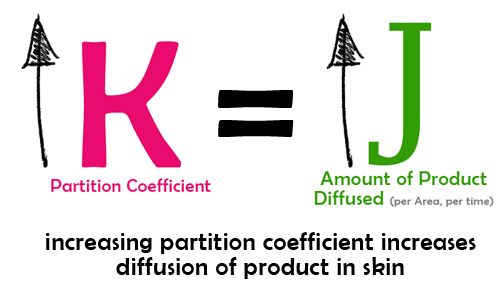
2. Parition Coefficient

We knew earlier that the parameter K is the partition coefficient and represents how the active substance is distributed between the stratum corneum membrane and the “vehicle” or carrier of the product. The use of solvents like ethanol in cosmetic products increases the partition coefficient thereby improving the penetration of active substances.

Cosmetic Insight #5: Have you noticed ethanol/denatured alcohol in some cosmetic products? Ethanol is a common solubilizer in cosmetic products. It solubilizes lipid in the stratum corneum making it more permeable[6]. However, long term and frequent use of products high in alcohol may lead to skin dryness. Since ethanol can cause reordering in the stratum corneum, it can decrease the skin’s capacity to retain moisture, because of compromised barrier.

Cosmetic Insight #6: Does your product sting during application? The partitioning of an active substance in the stratum corneum membrane is affected by pH or the acidity of cosmetic formulation. Chemical exfoliants like alpha-hydroxy acids for example, sting because they have low pH (acidic). A low pH will keep the partition coefficient high, so that product can penetrate the skin better.
Cosmetic Insight #6: Partition Coefficient may also answer the question at the beginning of this article. Two different products with exactly the same active ingredient may have different result on your skin. The type of penetration enhancers and solvents used in the formulation may vary, although they have the same active ingredients. This will significantly affect the performance of the product. However, skin type is an important factor too.
Remember:
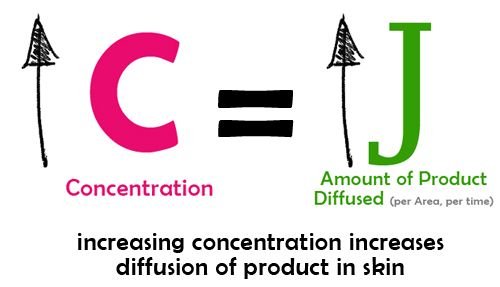
3.Concentration

This is perhaps the most common solution to enhance the penetration of active substances: increase its concentration in the formulation. However, formulations are limited to how well the active substance is tolerated by the skin.
Cosmetic Insight #7: Salicylic acid is a proven anti-acne substance. Its efficacy increases with higher concentration in the product. However, formulations are limited to small percentages because at above 4%, it may cause adverse reaction to the skin. Chemical Peels at very high concentration are performed by skin professionals.

Cosmetic Insight #8: Korean products has popularized essence and serums in skin cosmetic products. These products are concentrated because they designed to deliver active to the skin effectively.
Remember:
4. Path Length

Pathlength is the only parameter in Fick’s First Law that is inversely proportional to penetration of substance in the skin. It means that if you decrease the distance traveled by the substance to your skin, the greater is its rate of diffusion and the better will the product penetrate.

Cosmetic Insight #9: There is logic to the suggestion that exfoliating your skin regularly will help other cosmetic products to penetrate better in your skin. Physical Exfoliation (facial scrubs, gentle facial brush) and Chemical exofilation (alpha-hydroyx acids, salicyclic acids, etc) removes the superficial layers of the stratum corneum. This will thin out the barrier, so that other products can penetrate better.
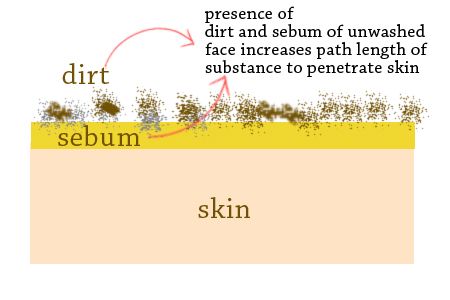
Cosmetic Insight #10: That’s why proper cleansing is necessary before applying any product in the skin. Skin that is not properly cleaned will have excess sebum and dirt that will make up an additional layer to the skin. This will increase L, the pathlength, and will affect the performance of other skin care products.
Wrapping Up
Fick’s First law of Diffusion is not a perfect description of the physical phenomenon but it gives us valuable insights about how products penetrate the skin. It also gives us a better understanding why product formulations cannot just contain all “sugar,spice and everything nice”to make one perfect product. For example, creating a nice emulsion with excellent skin-feel will not guarantee a good diffusivity through the stratum corneum. Formulators also need to consider how ingredients in the product can affect the diffusivity of other substances while keeping in mind its tolerability to the skin.
That's all for now guys. Oh Hey, Remember:
References:
[2] Draelos, Z (2016), Cosmetic Dermatology: Products and Procedures, 2nd ed., John Wiley & Sons, Ltd, West Sussex, UK
[3] Geankoplis,CJ (1993) Transport Processes and Unit Operations, 3rd Ed, Prentice Hall International, New Jersey
[4] Moser, K, et. Al (2001) Passive Skin Penetration Enhancement and its quantification in Vitro, Europe Journal of Pharmaceutics and Biopharmaceutics, Vol 52, pp 103-112.
[5] A Naik, L.A.R.M. Pechtold, R.O. Potts, R.H. Guy (1995) Mechanisms of oleic acid-induced skin penetration enhancement in vivo in humans. J Control. Release 37, pp 299
[6] Cartner, T et al (2017) Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A2 together with epidermal keratinocytes and skin irritation, International Journal of Cosmetic Science, Vol 39, Pp 188-196
[7] Iowata H. and Shimada K (2013) Formulas, Ingredients and Production of Cosmetics: Technologies of Skin and Hair Care Products of Japan, Hiroshi Iwata, Springer Tokyo
[8] Taoroian D, Lim, JE and Price PA (2007) The Size Exclusion Characteristics of Type I Collagen, The Journal of Biological Chemistry, Vol 292, pp 22437-22447
[9] Woo, JP, Misran M., Tan, LP (2014) Development of a Controlled Release of Salicylic Acid Loaded Stearic acid-oleic acid Nanoparticles in Cream for Topical Delivery, Scientific World Journal
Don't mind me.. just adding my upvote to anne's happiness.. :) I did try and learn about Fick and his laws, but why did he care so much about make up?? =p
Haha... It was originally used in medicine. Fick applied his knowledge in mathematics and physics to make a mathematical model applicable for heart physiology. ..
.
.
And the equation turned out to be very versatile as it explains diffusion of molecules in different membranes....even penetration of products in skin
ahhhhhhh. smart man. thanks for explaining it more..
And thank you for caring about my happiness.hhaha
:)
welcome to steemit,
Get to know how to earn more on your posts and get more followers.
Make new friends and interact.
We can share our thoughts.
In the steemit school, we have ultimate contest challenges where you get to win and make investments.
join the steemit school on discord https://discord.gg/pqWrzBn
Not a fan of the topic - cosmetics haha. But this is a great science post. Great way to merge your two passions and this is a really nice well-structured post.
Congrats on your 2nd curie vote. That's two in less than a month :D
Haha. Im sorry about that. I tried my best to make it uhmmm.....less boring.
What a great read! Everything can be broken down by learning the science behind it, love how you incorporate your fascination of science to cosmetics.
Such a well deserved curie!
Thank you @jan.lives!! I was actually scared that no one will even be interested in it...but I really enjoyed writing it.
I deal with Fick's law a lot in my work and sort of have tunnel vision on how it applies to nutrient diffusion to bugs in biofilms.
I never considered how it might apply to cosmetics. Nice coverage of both the science and education, thanks for writing this.
Hi @effofex thanks! I first came across fick's law as model for diffusing gases in permeable membranes. It's so beautiful ..how it applies to many fields of science.
.
.
Now you got me interested about your work
My whole life has just tumbled down before my eyes...the lies...
Hi @claritagigi... our blood vessels shrinks and dilates with varying temperature so cold temperature makes it appear smaller.
Also, pores are not the main route for skin care product. So we really shouldn't bother putting in extra effort in trying to "open or pores"