Role of reactive oxygen species in the pathophysiology of human reproduction
Role of reactive oxygen species in the pathophysiology of human reproduction
Cells living under aerobic conditions constantly face the oxygen (O2) paradox: O2 is required to support life, but its metabolites such as reactive oxygen species (ROS) can modify cell functions, endanger cell survival, or both. Hence, ROS must be continuously inactivated to keep only a small amount necessary to maintain normal cell function. Oxidative stress (OS) arises as a consequence of the excessive production of ROS and impaired antioxidant defense mechanisms. It is proposed that OS precipitates the range of pathologies that currently are thought to afflict the reproductive function. Recent reports have indicated that high levels of ROS are detected in semen samples of 25% to 40% of infertile men. The generation of ROS has become a real concern because of their potential toxic effects at high levels of sperm quality and function.
Spermatozoa are particularly susceptible to OS-induced damage because their plasma membranes contain large quantities of polyunsaturated fatty acids (PUFA) and their cytoplasm contains low concentrations of scavenging enzymes. Oxidative stress-mediated damage to the sperm plasma membrane may account for defective sperm function observed in a high proportion of infertility patients. Oxidative stress attacks not only the fluidity of the sperm plasma membrane but also the integrity of DNA in the sperm nucleus. Oxidative stress-induced DNA damage may accelerate the process of germ cell apoptosis leading to the decline in sperm counts associated with male infertility and the apparent deterioration of semen quality observed over the past 4 to 5 decades.

In recent years, the role of ROS in female infertility has been investigated extensively. Peritoneal, follicular, and hydrosalpingeal fluids represent important reproductive microenvironments. Given the fact that these fluids harbor gametes, zygotes, and cleaving embryos for variable durations, any abnormality in their chemical composition may have deleterious effects on the reproductive processes.
Although most of the mechanisms regulating ROS production and actions in both pathologic and physiologic cases remain to be elucidated, partial answers are now emerging. Elucidation of these mechanisms will enable researchers to create new, more effective methods to counteract the toxic effects of high levels of ROS and/or to improve fertility potential in assisted reproductive technologies. In this review, we summarize the efforts to explore the role of ROS in the pathophysiology of human reproduction.
Reactive oxygen species and sperm physiology
Until recently, ROS were exclusively considered toxic to the human spermatozoa. However, a strong body of evidence suggests that small amounts of ROS are necessary for spermatozoa to acquire fertilizing capabilities. The idea that limited amounts of ROS can intervene in a physiological manner in the regulation of some sperm functions was first evoked in a study by Aitken et al. Those investigators found that low levels of ROS can enhance the ability of human spermatozoa to bind with zonae pellucida, an effect that was reversed by vitamin E. Other studies have found that incubating spermatozoa with low concentrations of hydrogen peroxide (H2O2) stimulates sperm capacitation, hyperactivation, acrosome reaction, and oocyte fusion (14. Reactive oxygen species other than H2O2 such as nitric oxide and superoxide anion (O2•−) have also been shown to promote sperm capacitation and acrosome reaction.
Reactive oxygen species must be continuously inactivated to keep only a small amount necessary to maintain normal cell function. Interestingly, seminal plasma is well endowed with an array of antioxidant defense mechanisms to protect spermatozoa against oxidants. Antioxidants that are present in the seminal plasma compensate for the deficiency in cytoplasmic enzymes in the spermatozoa. Reactive oxygen species have a tendency toward chain reaction; that is, a compound carrying an unpaired electron will react with another compound to generate an unpaired electron, in such a manner that “radical begets radical.” Hence, the basic problem is to break this chain reaction by the formation of nonradical end products.
Chain-breaking antioxidants such as α-tocopherol (vitamin E) inhibit lipid peroxidation (LPO) in membranes by scavenging peroxyl (RO•) and alkoxyl (ROO•) radicals. The ability of α-tocopherol to maintain a steady-state rate of peroxyl radical reduction in the plasma membrane depends on the recycling of α-tocopherol by external reducing agents such as ascorbate or thiols. In this way, α-tocopherol is able to function again as a free radical chain-breaking antioxidant, even though its concentration is low. For efficient interception, the radical to be intercepted must have a relatively long half-life. The peroxyl radicals are major reaction partners because their half-life extends into the range of seconds (7 s).
In contrast, the hydroxyl radical (OH•), with its high reactivity and extremely short half-life (10−9s) cannot be intercepted with reasonable efficiency. Another line of antioxidant defense mechanisms is the prevention of excessive ROS formation. An example is the binding of metal ions, iron, and copper ions in particular, which prevents them from initiating a chain reaction.
Reactive oxygen species and male infertility
It has been reported that levels of antioxidants in seminal plasma from infertile men are significantly lower than those in plasma from controls. However, pathological levels of ROS detected in semen from infertile men are more likely a result of increased ROS production rather than reduced antioxidant capacity of the seminal plasma. In some situations, the damage caused by oxidants may be repaired. Unfortunately, spermatozoa are unable to repair the damage induced by excessive ROS because they lack the cytoplasmic enzyme systems that are required to accomplish this repair. This is one of the features that makes spermatozoa unique in their susceptibility to oxidative insult.
Virtually every human ejaculate is contaminated with potential sources of ROS. It follows that some spermatozoa will incur oxidative damage and a concomitant loss of function in every ejaculate. Thus, the impact of ROS on male fertility is a question of degree rather than the presence or absence of the pathology. A study by Fisher and Aitken has indicated that male germ cells at various stages of differentiation from pachytene spermatocytes to mature caudal epididymal spermatozoa from mature male rats, mice, hamsters, and guinea pigs have the potential to generate ROS. Clear evidence also suggests that human sperm can produce ROS.
Spermatozoa may generate ROS in two ways: the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system at the level of the sperm plasma membrane and the NADH-dependent oxidoreductase (diaphorase) at the level of mitochondria. The mitochondrial system is the main source of ROS in spermatozoa from infertile men. Gomez et al. have indicated that levels of ROS production by pure sperm populations were negatively correlated with the quality of sperm in the original semen. High levels of ROS production in human ejaculates may originate from morphologically abnormal spermatozoa and/or seminal leukocytes. The link between poor sperm quality and increased ROS generation lies in the presence of excess residual cytoplasm (cytoplasmic droplet).
When spermatogenesis is impaired, the cytoplasmic extrusion mechanisms are defective, and spermatozoa are released from the germinal epithelium carrying surplus residual cytoplasm. Under these circumstances, the spermatozoa that are released during spermiation are thought to be immature and functionally defective. Retention of residual cytoplasm by spermatozoa is positively correlated with ROS generation via mechanisms that may be mediated by the cytosolic enzyme glucose-6-phosphate dehydrogenase (G6PD). Huszar and Vigue have found that sperm morphological irregularities are significantly correlated with high creatine kinase (CK) activity in human spermatozoa. Similarly, recent studies have found an inverse relationship between CK levels and sperm morphological forms and have suggested that CK levels can be used as a reliable marker for sperm quality and fertilizing potential in subfertile men. A positive relationship was found between CK activity and the rate of lipid peroxidation, as measured by malonaldehyde (MDA) formation, in sperm fractions separated by Percoll, as per Huszar and Vigue.
Recent studies by Ollero et al. (39 and Gil-Guzman et al. have shown that levels of ROS production in semen were negatively correlated with the percentage of normal sperm forms as determined by the World Health Organization classification and by the strict criteria of Kruger et al.. In the same studies, the authors found significant variation in levels of ROS production in subsets of spermatozoa at different stages of development. After Isolate gradient fractionation of ejaculated sperm, ROS production was found to be highest in the immature sperm fraction (containing sperm with abnormal head morphology and cytoplasmic retention) and lowest in the mature sperm fraction (containing normal-looking motile sperm) and in the immature germ cells.
The relative proportion of ROS-producing immature sperm was directly correlated with nuclear DNA damage values in mature sperm and inversely correlated with the recovery of motile, mature sperm. These interesting findings led to the hypothesis that oxidative damage of mature sperm by ROS-producing immature sperm during their comigration from seminiferous tubules to the epididymis may be an important cause of male infertility.
Studies from our institution also indicated that the production of ROS by human spermatozoa increased significantly when spermatozoa were exposed to repeated cycles of centrifugation. The duration of centrifugation was found to be more important than the force of centrifugation for inducing ROS formation by human spermatozoa. These data are important because exposing spermatozoa to high levels of ROS may cause DNA fragmentation, which may have adverse consequences if they are used for assisted reproductive techniques (ARTs). This may be true particularly in light of a recent report by Zini and co-workers, who found that the improvement in sperm motility after Percoll processing was not associated with a similar improvement in sperm DNA integrity. Those investigators recommended that the current sperm preparation techniques be reevaluated with the goal of minimizing sperm DNA damage.
Role of leukocytospermia in excessive ROS production in semen
Leukocytes are present throughout the male reproductive tract and are found in almost every human ejaculate. However, the clinical significance of increased leukocyte infiltration in semen, that is, leukocytospermia, is currently the subject of controversy. On one hand, leukocytospermia has been linked with poor sperm quality, reduced sperm hyperactivation, and defective sperm function. On the other hand, no correlation was found between seminal leukocyte concentrations and impaired sperm quality or defective sperm function. The World Health Organization (WHO) defines leukocytospermia as the presence of peroxidase-positive leukocytes in concentrations of ⪢1×106 per milliliter of semen.
Peroxidase-positive leukocytes include polymorphonuclear leukocytes, which represent 50% to 60% of all seminal leukocytes, and macrophages, which represent another 20% to 30%. Peroxidase-positive leukocytes in semen are contributed largely by the prostate and the seminal vesicles. Peroxidase-positive leukocytes were found to be the major source of high ROS production in semen. Activated leukocytes can produce 100-fold higher amounts of ROS than nonactivated leukocytes. Leukocytes may be activated in response to a variety of stimuli including inflammation and infection. Activated leukocytes increase nicotinamide-adenine dinucleotide phosphate (NADPH) production via the hexose monophosphate shunt.
The myeloperoxidase system of both polymorphonuclear leukocytes and macrophages is also activated, which leads to respiratory burst and production of high levels of ROS. Such an oxidative burst is an early and effective defense mechanism for killing microbes in cases of infection. Sperm damage from ROS that is produced by leukocytes occurs if seminal leukocyte concentrations are abnormally high, such as in leukocytospermia, or if seminal plasma was removed during sperm preparation for assisted reproduction.
However, Sharma et al. observed that seminal leukocytes might cause OS even at concentrations below the WHO cutoff value for leukocytospermia. This may be due to the fact that seminal plasma contains large amounts of ROS scavengers but confers a very variable (10% to 100%) protection against ROS generated by leukocytes. The lack of clinical significance of leukocytospermia reported in some studies is possibly a reflection of the powerful antioxidant properties of the seminal plasma, which may provide protection against leukocyte-mediated oxidative stress (OS).
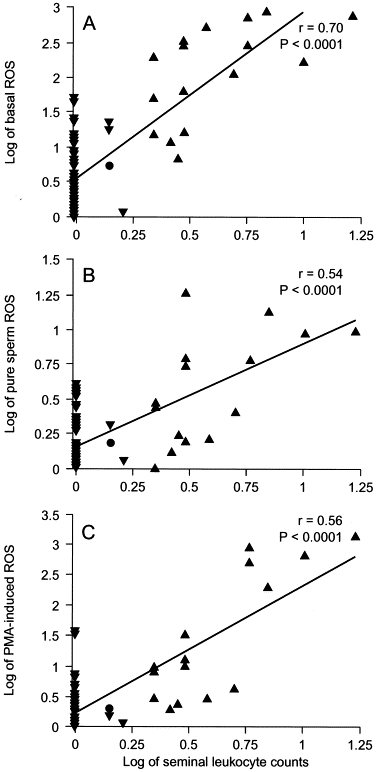
It is unclear from the existing literature whether the interaction between leukocytes and spermatozoa implies a direct or indirect stimulatory effect, which may enhance the capacity of spermatozoa to generate excessive ROS. Recent data from our center indicated that levels of ROS production by pure sperm suspensions from infertile men with a laboratory diagnosis of leukocytospermia were significantly higher than were those from infertile men without leukocytospermia (Table 1). In addition, seminal leukocyte concentrations were strongly correlated with levels of ROS in the original cell suspensions containing sperm and leukocytes (basal ROS); in the leukocyte-free sperm suspensions (pure sperm ROS); and in the leukocyte-free sperm suspensions (phorbol ester–induced ROS; Fig.1 ). This observation led to the postulation that seminal leukocytes play a role in enhancing sperm capacity for excessive ROS production either by direct sperm–leukocyte contact or by soluble products released by the leukocytes.
TABLE 1
Basal ROS levels in original cell suspensions (containing sperm and leukocytes) and in pure sperm suspensions (leukocyte-free sperm suspensions after complete removal of leukocytes using anti-CD coated paramagnetic beads) in three study groups.
Values are median (25th, 75th percentiles). ROS is measured by chemiluminescence assay.
Wilcoxon rank-sum test was used for comparison, and statistical significance was assessed at P < .05 level.
FIG 1
Correlation of seminal leukocyte concentrations with the following: levels of basal ROS (A); pure sperm ROS (B); and PMA-induced ROS in donors (•), non-leukocytospermic patients (▾), and leukocytospermic patients (▴; C). Basal ROS = levels of ROS in washed sperm suspensions after a simple wash and resuspension in phosphate-buffered saline (containing sperm and leukocytes); pure sperm ROS = levels of ROS in leukocyte-free sperm suspensions after complete removal of leukocytes using anti-CD45–coated paramagnetic beads. PMA-induced ROS = levels of ROS in leukocyte-free sperm suspensions after stimulation of phorbol 12-myristate 13-acetate. Seminal leukocyte concentrations (×106/mL) and levels of ROS (×106 counted photons per minute per 20×106 sperm/mL) were log transformed.
This new observation may have significant implications for the fertility potential of sperm both in vivo and in vitro. Excessive production of ROS by sperm in patients with leukocytospermia implies that both the free radical–generating sperm themselves and any normal sperm in the immediate vicinity will be susceptible to oxidative damage. Furthermore, once the process of LPO is initiated, its self-propagating nature ensures a progressive spread of the damage throughout the sperm population.
Pathological effects of increased ROS: the concept of OS
Generally, the term OS is applied when oxidants outnumber antioxidants, when peroxidation products develop (60, and when these phenomena cause pathological effects. In the context of human reproduction, excessive ROS production that exceeds critical levels can overwhelm antioxidant defense strategies of spermatozoa and seminal plasma, causing OS. All cellular components including lipids, proteins, nucleic acids, and sugars are potential targets for OS. The extent of OS-induced damage depends not only on the nature and the amount of ROS involved but also on the moment and duration of ROS exposure and on extracellular factors such as temperature, oxygen tension, and the composition of the surrounding environment (e.g., ions, proteins, and ROS scavengers).
Lipid peroxidation of sperm plasma membrane
Lipid peroxidation is broadly defined as “oxidative deterioration of PUFA,” which are fatty acids that contain more than two carbon-carbon double bonds. The LPO cascade occurs in two fundamental stages: initiation and propagation. The hydroxyl radical (OH•) is a powerful initiator of LPO. Most membrane PUFA have unconjugated double bonds that are separated by methylene groups. The presence of a double bond adjacent to a methylene group makes the methylene carbon-hydrogen bonds weaker, and therefore hydrogen is more susceptible to abstraction. Once this abstraction has occurred, the radical produced is stabilized by the rearrangement of the double bonds, which form a conjugated diene radical that can then be oxidized.
This means that lipids, which contain many methylene-interrupted double bonds, are particularly susceptible to peroxidation. Conjugated dienes rapidly react with O2 to form a lipid peroxyl radical (ROO•), which abstracts hydrogen atoms from other lipid molecules to form lipid hydroperoxides (ROOH). Lipid hydroperoxides are stable under physiological conditions until they contact transition metals such as iron or copper salts. These metals or their complexes cause lipid hydroperoxides to generate alkoxyl and peroxyl radicals, which then continue the chain reaction within the membrane and propagate the damage throughout the cell. Propagation of LPO depends on the antioxidant strategies employed by spermatozoa. One of the by-products of lipid peroxide decomposition is MDA.
This by-product has been used in biochemical assays to monitor the degree of peroxidative damage sustained by spermatozoa. The results of such an assay exhibit an excellent correlation with the degree to which sperm function is impaired in terms of motility and the capacity for sperm-oocyte fusion.

Go here https://steemit.com/@a-a-a to get your post resteemed to over 72,000 followers.
What a wonderful post, so informative. Kindly reference the page you got some of the info. Thanks for sharing
Thanks. I will do so.
Congratulations!,@solomon507 Your post has been upvoted by @reachout
Our goal is to support Minnows on Steemit.
Join our discord group https://discord.gg/NWAkKfn
Major Upvote Sponsor : @bleepcoin
SP Donation by @rufans & @solomon507
Join Our Trail here: https://steemauto.com/dash.php?i=15&id=1&user=reachout
Very detailed and informative. ROS causes a lot of problems to our body and we need anti-oxidants to counter those negative effects. Great article with a lot of explanation. Keep up the good work !
Thanks for taking your time to read. We definitely need anti-oxidants.