Which Liquids Freeze And Why? Fun Experiment For Kids!
Looking for a fun activity to do with the kids on a rainy day? How about giving this simple yet really cool experiment a go? Kids can learn while having fun and passing time. This activity can be done at home or as a group at school - the more the merrier actually! The suggested age group for the experiment would be from 4-13 (some older kids might still get a kick out of it too).
This science experiment is a great way to learn about the properties of solids and liquids and how they change at different temperatures (hot and cold). If you are working with older children, they might learn more if they have an opportunity to list the liquids/solutions they are going to use in their experiment so that they can make a note of what state they are in at varying temperature, for example: room temperature (in it's natural state), in a hot environment (out in the sun or placed in a heated oven) and then at freezing point. They can also make a note of which substance melts first etc. and which substance freezes faster or perhaps does not freeze at all. These notes can be used for further analyses and discussions.

Focusing on seeing which substances freeze and at what speed, you will need to gather the following materials:
- Access to a freezer
- An ice tray
- A timer
And a variety of the following liquids/substances:
- Water
- Dish washing liquid
- Honey
- Salt water solution
- Vinegar
- Milk
- Cooking Oil/Hand Sanitiser
Let's get going!
Before you fill your trays, mark each "cube" with a symbol so that you know what substance is in each cube, water and vinegar can look the same for example. Fill each of the ice cubes with one of the above listed substances, making sure that they are all set at room temperature before you start. Once the tray has been filled, place it flat into the freezer. Get the kids to list each of the substances in a notebook, or to start compiling a graph (up to you how you want them to capture the data) while they wait. Set a timer to go of every 15 minutes to observe the tray to see which substances have started freezing first and which ones are not freezing at all.
To help pass the time, you could take the same substances and heat them, to see what happens to the properties in a hot environment, this way they can compare the data.

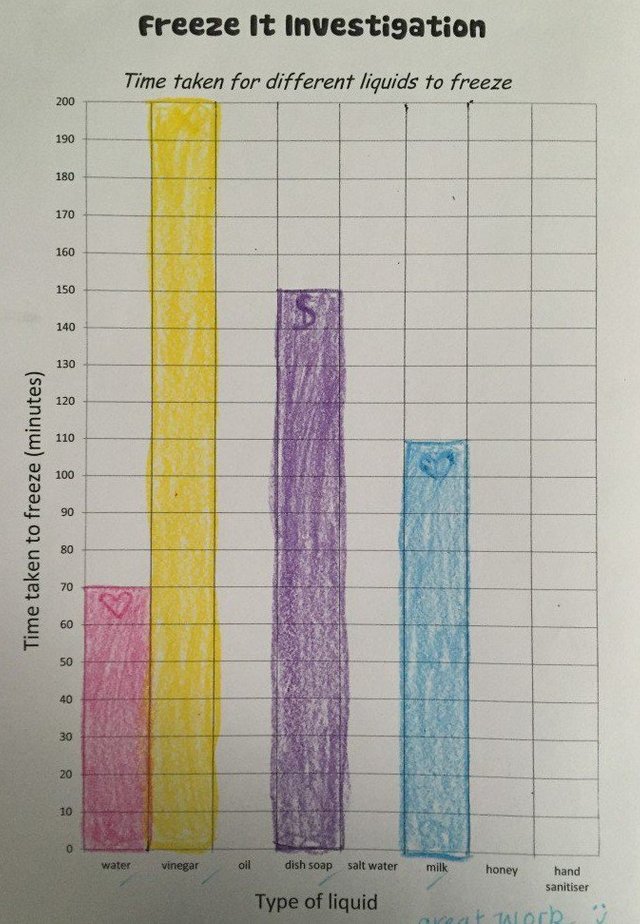
The findings
Before discussing the findings, you might want to teach the kids the meaning behind the words "viscous", since the viscosity of each liquid/solution comes into play here.
Viscous means sticky, gluey and syrupy. So if something is viscous, you usually don't want to stick your fingers in it — that goes for boogers and maple syrup alike. -https://www.vocabulary.com/dictionary/viscous
The findings will show that the liquids with a lower viscosity froze faster while those that where thicker took the longest to freeze, or didn't freeze at all!
Milk freezes the fastest, this is because there are already solids suspended in milk, making it nearer to a solid state than water and vinegar for instance (both of which froze soon after the milk). The liquids that had a higher viscosity, like the dish soap, honey, hand sanitiser and oil, did not freeze at all - this is because there is more dissolved solids in the water than there is water.

Did you know?
Saltwater has a much lower freezing point (the freezing point is the temperature where something freezes) than freshwater does. And the more salt there is in it, the lower the freezing point gets. So in order to know the exact temperature that it’s going to freeze, you have to know just how salty it is. For saltwater that’s as saturated as it can possibly get (i.e. there’s no way to dissolve any more salt in it no matter how hard you tried), the freezing point is -21.1 degrees Celsius. This is when the saltwater is 23.3% salt (by weight) - https://van.physics.illinois.edu/qa/listing.php?id=1722
Source: http://ledascience.edublogs.org/2015/11/16/freeze-it/
I love experiments like these, they are so simple and can be done at home or in the classroom without much fuss at all, and the opportunity to learn new things is vast, for older kids and even for toddlers who are just beginning to understand the world. ENJOY!
 Team South Africa banner designed by @bearone
Team South Africa banner designed by @bearone
Thanks, great experiment to try at home. Loved playing with such things when I was young especially making all different kind of rockets with our chemistry set!!
I know, there are so many "basic" experiments that are actually super fun to do! Rockets sound like FUN!
Thanks for the ideas! Very interesting post!
Thank you :)
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by sweetpea from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews/crimsonclad, and netuoso. The goal is to help Steemit grow by supporting Minnows and creating a social network. Please find us in the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.