How To Read A Graduated Cylinder

Image Credit: Xavax [Public domain], from Wikimedia Commons
This post isn’t intended to blind anyone with SCIENCE. It really isn’t aimed at readers who are accustomed to doing scientific research - and OMAHGOSH! Some of you blow my mind with the research you share. There is some top notch science information posted to Steemit. Instead, it is meant to address a very common problem that I encounter with students in my high school science classes during science labs. So, if you’re in the mood for a light-hearted wade through the shallow end of the science pool, then please read on as we discuss…
How To Read A Graduated Cylinder
I taught 7th grade science for nearly a decade. It was fun; it was pretty easy; and I had it down to the point where I could walk in on any given day and know what I needed to teach without hours of planning. But, all good things must come to an end; so, near the end of last year, my principal asked me if I would be interested in moving up to teach ninth grade. I felt honored by the request, so I obliged (a good move, by the way), and I now have a lot of my former seventh grade students again in my ninth grade classes.
Now, I know for a fact that we talked about the importance of precision in our measurements during lab. I remember teaching them what a graduated cylinder is, what it looks like, why we would use one, and how to use it. You wouldn’t know it from watching them this year, though.
I explain to them before just about every lab that if the procedure says “pour approximately XX mL of liquid into a beaker”, then just get it close. However, if it doesn’t say approximately, then they are to assume that it means exactly XX mL of liquid. Then, I ask them, “What instrument would we use to measure an exact quantity of a liquid?” Each time I ask the question, I do get a few more students who respond correctly, but never close to 100%.
Then, we go into the lab, and I hear the question, “In the procedure, it says to pour 125 mL of water into the beaker, but there’s not a line for 125?” I know that statement isn’t formatted as an interrogative, but that little voice inflection that they add at the end followed by a pause makes it clear that they want me to give them an answer. My response: What instrument would you use to measure the exact quantity of a liquid?” “Oh, yeah!”, they say; and I move on to the next group. Of course, when I look back, I usually see something like this:

“Holdon, holdon, hold. on. How much water is in that graduated cylinder?”
“125 mL.”
“How about now? as lift the cylinder higher into the air and then lower it.
”I can’t tell.”
“Why not?”
“Because it is at an angle.
“So, what do you think you need to do?”
“Get on the same level as the water?”
“There ya’ go!”, as I triumphantly walk away.
I look back to see something like this:

”Are you holding that level?”
*Yeah.”
Are ya’ really, though?
This is usually the part where the lab partner goes,
”You have to sit it down on the table. Dummy! Watch.”
Followed by their attempt at using this seemingly advanced alien technology. When they have finished:
”How much did you put in there?”
”125 mL.”
”Are you sure? Because, I only see a little over 124.”
”Huh?”
”Did you read it at the meniscus?”
”What’s a meniscus?”
What Is A Meniscus?
Most liquids, when poured into a container, will form a meniscus. This occurs due the properties of adhesion and cohesion. To put it simply, adhesion is the tendency of a substance to stick to a different substance, while cohesion is the tendency of a substance to stick to itself. Water sticks to itself pretty well due to the hydrogen bonds that form between each molecule; but, when it comes into contact with glass, it prefers to cling to the surface of the glass. As a result, the water molecules will piggyback on each other in an attempt to adhere to the glass.In a cylindrical container, this means that the water is migrating outward (away from the center) in all directions, leaving a shallow spot in the middle - a phenomenon that we call a meniscus.
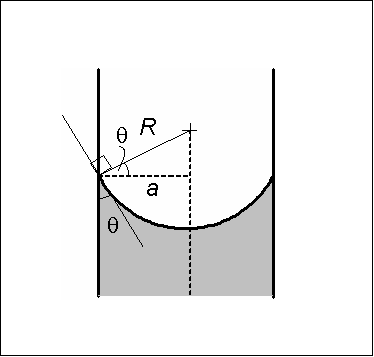
Image Credit: Spherical Meniscus by Cutler
Reading The Meniscus
When reading a graduated cylinder, you are supposed to look at the center (the lowest point) of the meniscus. To do this, you must place your graduated cylinder on a flat, level surface and bring yourself to eye level with the liquid’s surface. Find the lowest point of the water’s surface and compare it to the graduation marks on the side of the cylinder. If it lies between two marks, then you should estimate out to one decimal place farther than the instrument reads. For example, the following graduated cylinder contains ~17.8 mL of water.
But, There’s A Twist!
Obviously, water forms a concave shaped meniscus in a glass graduated cylinder. But, did you know that there are some liquids that form a convex meniscus in a glass graduated cylinder? Take liquid mercury for example.
Image CreditImage by karabekirus
It turns out that the shape of the meniscus is the result of a battle between the cohesive forces holding the liquid together and the adhesive forces pulling it toward the graduated cylinder’s surface. In the case of water, the adhesive forces are stronger, so the water is pulled out toward the edges. In mercury, the cohesive forces are a lot stronger than the adhesive forces, so mercury is pulled from the edges inward toward the center.
When reading a graduated cylinder with a convex meniscus, you will still read it at the center-most point in the liquid; only in this case that will be at its highest point.
So, There You Have It, Folks!That is how you read a graduated cylinder. And, if you’re one of my students reading this because I assigned this to you in class, Go to Google Classroom and take the quiz!
Resources
How to Read a Meniscus in Chemistry
chem.libretexts.org
This Physics Forums Discussion
This Reddit Discussion
.jpg)
This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @curie.
If you appreciate the work we are doing then consider voting both projects for witness by selecting stem.witness and curie!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!