Chemistry and Metabolism of Biomolecules #9: Metabolism of Glucose (Part 2).
Introduction
 Glucose has shown a lot of diversity in biological systems (License: Public Domain]:
Pixabay
Glucose has shown a lot of diversity in biological systems (License: Public Domain]:
Pixabay The previous installment of this series showed how glucose could be broken down via glycolysis to yield energy in the form of ATP. Glucose is a very versatile molecule and it would interest you to know that it is not always needed by cells solely for the energy it can provide. Sometimes it is needed for other molecules it can be converted into. Molecules which are relevant to cells.
These molecules are specifically NADPH and Ribose-5-phosphate.
The cells are in constant need of these molecules for various biochemical processes like free radical scavenging and nucleic acid biosynthesis. A special pathway which was briefly mentioned in the previous installment of this series provides an avenue for these molecules to be synthesized and presented for effective utilization by these cells.
This pathway is known as the pentose phosphate pathway. It is generally considered an alternative pathway for glucose oxidation. Taking a systematic look at the name of this pathway, it can be seen that pentose phosphates production is the main goal of this pathway. Rightfully so I will say. But not entirely correct, as NADPH production is also the goal of this pathway.
The Pentose Phosphate Pathway: An Alternative Route Glucose can follow.
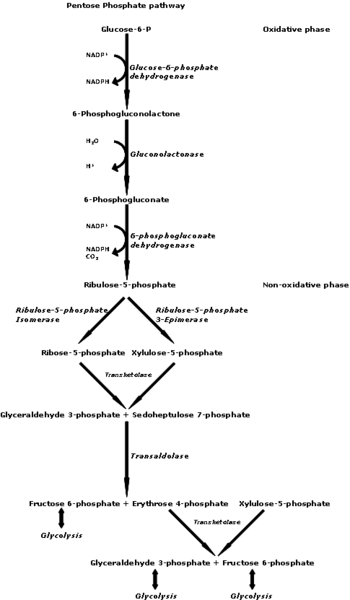 The Pentose Phosphate Pathway: A schematic representation [License: CC-BY-SA 3.0, Author: GdenBesten]:
Wikicommons
The Pentose Phosphate Pathway: A schematic representation [License: CC-BY-SA 3.0, Author: GdenBesten]:
Wikicommons The pentose Phosphate pathway provides a means for glucose to be converted to ribose-5-phosphate with the production of NADPH. Ribose-5-Phosphate is an important molecule in nucleic acid synthesis. This is why ribose-5-phosphate can be synthesized by almost every cell in the human body since every human cell needs to possess a DNA. Ribose-5-Phosphate is in itself a pentose phosphate (pentose = 5 carbon sugar e.g Ribose & Ribulose) and being the major product in the pentose phosphate pathway (PPP), the PPP has had to be called what it is called. A pentose phosphate is a 5 carbon sugar molecule that has a phosphate group attached to either one of its carbon units. In technical sense, we can have Ribose-1-phosphate since any of ribose carbon units can be phosphorylated.
The PPP has also been called the hexose monophosphate shunt (HMP shunt) since Glucose-6-phosphate is produced as an ultimate product in the second phase of the pathway. Glucose is a hexose (six carbon) Sugar, thus, the alternative name for this pathway.
Cells have developed various means of regulating various pathways especially those that have common intermediates. I mentioned compartmentalization in the post where I talked about Ketolysis and Ketogenesis. I showed how important it was for Ketogenesis to take place in the mitochondria to prevent cholesterogenesis (which takes place in the cytosol) from getting in the way as they both share a common intermediate: HMG coA.
The glycolytic pathway is supposed to get in the way of the pentose phosphate pathway too as they both have glucose-6-phosphate as a common intermediate. This is hugely due to the fact that both pathways occur in the same compartment; the cytosol. This doesn’t happen anyway.
So how does the cell make it possible for both pathways (glycolysis and PPP) to occur when necessary without making use of compartmentalization
The cells have special means to detect the NADPH/NADP+ ratio in the body which is instrumental in knowing when glycolysis or PPP should take place. When the NADPH/NADP+ ratio is high, glucose is used more via the glycolytic pathway as this implies that NADPH is present in sufficient amounts and there is therefore no need for its further production.
When the NADPH/NADP+ ratio is low, glucose is utilized more via the pentose phosphate pathway as this implies that there is a low concentration of NADPH and a high concentration of NADP+ which is needed for the production of NADPH.
How does the pentose phosphate pathway proceed biochemically ?
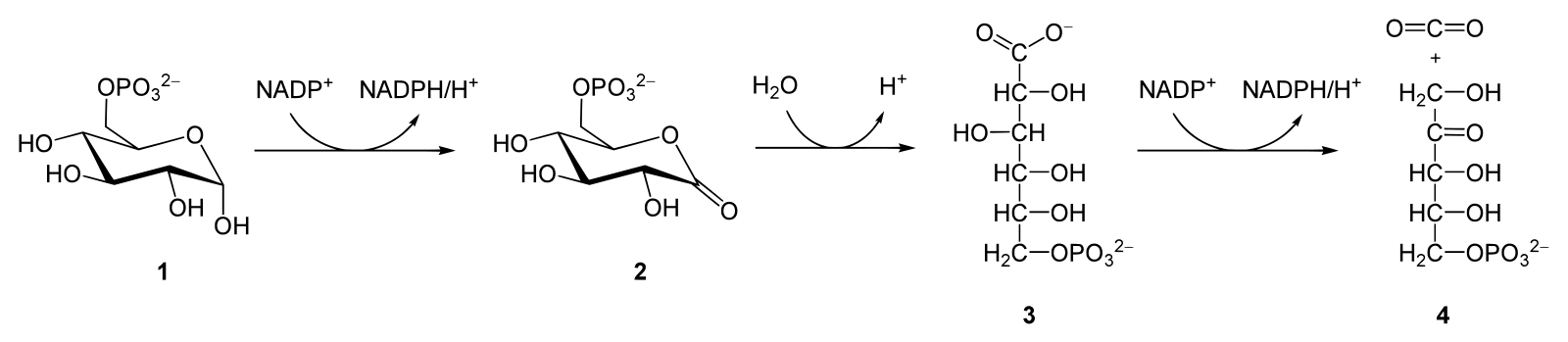 The oxidative phase of the pentose phosphate pathway (License: Public Domain]:
Wikicommons
The oxidative phase of the pentose phosphate pathway (License: Public Domain]:
Wikicommons 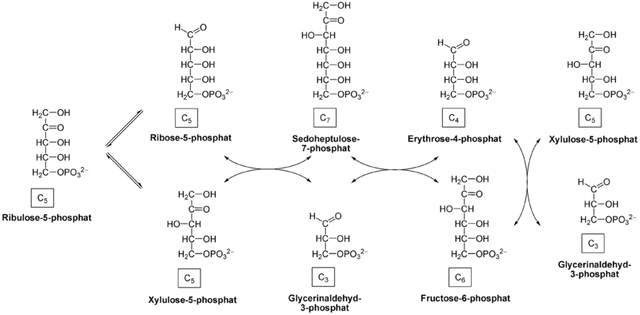 The non-oxidative phase of the pentose phosphate pathway [License: CC-BY-SA 3.0, Author: Yikrazuul]:
Wikicommons
The non-oxidative phase of the pentose phosphate pathway [License: CC-BY-SA 3.0, Author: Yikrazuul]:
Wikicommons Biochemists like to look at the PPP in two parts. The first part is considered the oxidative irreversible phase while the second part is considered the non-oxidative reversible phase.
The oxidative phase starts with the conversion of glucose-6-phosphate to 6-phosphogluconolactone catalyzed by the enzyme glucose-6-phosphate dehydrogenase. This is the first point where NADPH is produced and surprisingly, it is a reversible reaction even though the whole pathway is considered irreversible in a biochemical sense.
The second step in this pathway involves the hydrolysis (breaking a bond using a water molecule) of the already formed 6-phosphogluconolactone to 6-phosphogluconate. The main enzyme here is a lactonase.
The 6-phosphogluconate loses a hydrogen atom and a CO2 molecule leading to the formation of Ribulose-5-phosphate. This is termed oxidative decarboxylation and is also seen where pyruvate is converted to acetyl coA in the citric acid cycle. This Step is catalyzed by 6-phosphogluconate dehydrogenase. There is also a formation of NADPH here.
The final step in the oxidative phase of the PPP is the conversion of ribulose-5-phosphate to ribose-5-phosphate. An isomerase (phosphopentose isomerase) comes into play here. Ribose-5-phosphate and Ribulose-5-phosphate are structural isomers. This is where the pathway ends for most tissues, specifically tissues that need to produce nucleotides.
Tissues that need NADPH in abundance convert this ribulose-5-phosphate to glucose-6-phosphate ultimately so as to get more NADPH. This constitutes the second part of the PPP; The reversible non-oxidative phase of the PPP. This is basically an avenue for pentose phosphate sugars to be recycled to hexose monophosphate sugars.
The ribulose-5-phosphate is converted to xylulose-5-phosphate acted upon by ribose-5-phosphate isomerase. This newly formed xylulose-5-phosphate donates two carbon atoms to ribose-5-phosphate leading to the formation of sedoheptulose-7-phosphate and Glyceraldehyde-3-phosphate. Transketolase (an enzyme that orchestrates the transfer of a two carbon unit from a ketose Sugar to an aldose Sugar) is the enzyme responsible here.
Transaldolase comes into play in the next step. This enzyme catalyzes the transfer of a three carbon unit from an aldose Sugar to a ketose Sugar. A three carbon unit is transferred from sedoheptulose-7-phosphate to Glyceraldehyde-3-phosphate leading to the formation of fructose-6-phosphate and erythrose-4-phosphate.
The formed fructose-6-phosphate can be acted upon by phosphohexose isomerase leading to the formation of glucose-6-phosphate which is used in the synthesis of more NADPH molecules (The sole purpose of having to go through this phase).
The erythrose-4-phosphate can accept two carbon units from another xylulose-5-phosphate molecule catalyzed by transketolase leading to the formation of fructose-6-phosphate (which is converted to glucose-6-phosphate) and Glyceraldehyde-3-phosphate. The latter molecule can go into the gluconeogenic pathway leading to the formation of glucose-6-phosphate. It’s pretty obvious how everything occurs in the direction of glucose-6-phosphate production so as to generate as much NADPH as possible.
Glucose-6-phosphate Dehydrogenase Deficiency: A Reason Pythagoras Wouldn’t Eat Falafels, People in the Tropics Don’t Die Rampantly Due To Malaria and The AntiMalarial Drug, Premaquine is No Longer in Use.
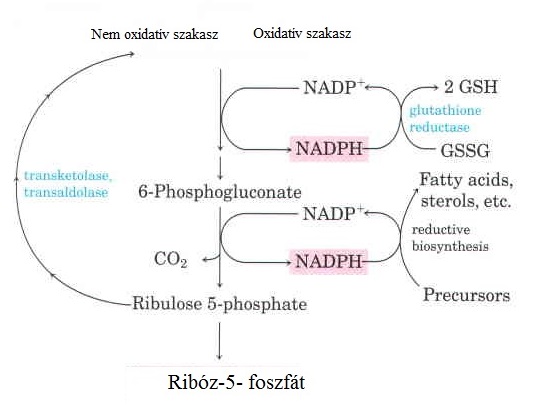 How NADPH is vital in reducing glutathione [License: CC-BY-SA, 4.0, Author: Flora5]:
Wikicommons
How NADPH is vital in reducing glutathione [License: CC-BY-SA, 4.0, Author: Flora5]:
Wikicommons The NADPH produced in the oxidative phase of PPP is actually more important than one could imagine. It is an absolute requirement in fatty acid synthesis, nucleic acid synthesis, neurotransmitter synthesis as well as cholesterol biosynthesis.
It’s most important role is in the reduction of glutathione which is necessary for the mopping up of free radicals in the body. NADPH is produced in the first and third step of the oxidative phase of the PPP. The first step has to occur before the third step occurs so a deficiency in the enzyme necessary for the first step will lead to no production of NADPH.
Glutathione is an antioxidant. It must be continually kept in a reduced state so it can easily combine with free radicals (the reactive oxygen species) reducing oxidative stress in cells. NADPH is necessary in keeping glutathione in a reduced state.
In glucose-6-phosphate dehydrogenase deficient individuals, glutathione is in an oxidized state since no NADPH is available to reduce it and so free radicals can cause oxidative stress as they please. They cause oxidative stress by reacting with lipids which are integral components of cell membranes altering these membranes and making them more porous and weaker. This is generally known as Lipid Peroxidation.
 Pythagoreans [License: Public Domain]:
Wikicommons
Pythagoreans [License: Public Domain]:
Wikicommons Pythagoras refused to eat falafel cause they made him sick. This was so because falafel contains fava beans as an ingredient. Fava beans themselves contain pamaquine as an active compound and this compound is capable of generating free radicals like the peroxides which can damage cell membranes.
Pythagoras actually stopped his followers from eating falafel since it was noticed that people who consumed falafels presented with Jaundice, and in a few cases, kidney failure. This was due to the fact that these individuals were glucose-6-phosphate dehydrogenase deficient (which Pythagoras was ignorant of) so consumption of falafel led to the lysis of their red blood cells due to the attack on the cell membranes of these RBCs by free radicals resulting in oxidative stress. Their red blood cells were at the mercy of reduced glutathione which was unavailable due to the absence of NADPH so these symptoms had to suffice. These individuals were described as having favism.
The antimalarial drug premaquine actually destroyed the malaria parasite, plasmodium falciparum by creating oxidative stress within RBCs. This led to similar symptoms as seen in people who consumed fava beans. People who were not deficient of the enzyme G-6-P dehydrogenase had no cause for worry but the people who were, had every reason to worry.
People who lack the enzyme do not have glutathione in the reduced state. Reduced glutathione binds to haemoglobin molecules keeping them in reduced states and preventing them from cross linking. Cross linking of haemoglobin results in the formation of Heinz Bodies which can damage red blood cell membranes and make them more prone to lysis. This is why the use of this drug as an antimalarial agent was primarily discontinued.
G-6-P dehydrogenase deficiency is not particularly lethal unless combined with certain environmental factors. As a matter of fact, it offers resistance to malaria caused by the plasmodium falciparum parasite. This parasite is particularly sensitive to oxidative stress so the oxidative stress seen in G-6-P dehydrogenase deficiency kills the parasite ultimately. Natural selection has actually led to individuals in the tropics maintaining the G-6-P dehydrogenase deficient genotype conferring malarial resistance on these individuals.
This is to say Pythagoras actually jinxed it and prevented his followers who had normal glucose-6-phosphate dehydrogenase activity from gaining resistance to malaria by discouraging them from eating falafels. They would most likely die of malaria.
Summary
Glucose has shown versatility as buttressed in this installment. Upcoming installments will even go further to show its versatility. The glycolytic and pentose phosphate pathway has been shown to have an intersecting point via a common intermediate. We have also seen how deficiency of glucose-6-phosphate dehydrogenase can lead to several metabolic derangements and of course, why Pythagoras wouldn’t eat falafel. Till next time, I hope you left here knowing a little more than you did before coming here.
References
Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2000). Lehninger principles of biochemistry. New York: Worth Publishers. pp. 549-555.
Berg, J. M., TyMoczko, J. I & Stryer, L. (2002). Biochemistry (5th Edition). New York. W.H. Freeman. pp. 844-857.
Murray, R. K., Granner, D. K., Mayes, P. A. and Rodwell, V. W. (2003). Harper’s Illustrated Biochemistry. (26th Edition). Lange Medical Books. New York. pp. 163-166.
Image Sources
All images are from pixabay and wikicommons licensed under creative commons and eligible for commercial use.
I'm a proud member of the steemstem community which promotes quality posts in the science, technology, engineering and mathematics fields on the steem blockchain mainly through interaction and engagement. Feel free to join us on discord here
I think we should even say glucose is the one sustaining the body since it is highly needed by the body not only for the production of ATP (the energy legal tender in the body) but also for the synthesis of other biomolecule in the body. Well done @kingabesh .
yes man. that is correct
Well pretty accurate and interesting as always, had to leave a comment for the great work you did, keep it up, need more Pythagoras facts!! =)
Thanks :). I’m glad you found it interesting.
To listen to the audio version of this article click on the play image.

Brought to you by @tts. If you find it useful please consider upvote this reply.
This is really cool
I really don't know what to say because it's not my field but I would just say keep doing this you are doing.... I love it
im glad you liked it and sure, i will
Ohhh, i do not know much about Biochemical pathways, but when i read an educative post, i know.
Kudos
thanks man
I've always had issues understanding metabolism on a molecular scale. And although even the pronunciation of those complex molecular names wear me out more often than not, your candid explanation of those processes makes quite a lot of sense. Thanks for helping out in this regard!
Thank you for finding this useful and more importantly for the honest comment. I’m glad you found this helpful
You're very welcome :)
@kingabesh Good work.
Good work. Technically updating and enlightening.
oh thanks a lot man. i'm glad you found it enlightening