Nitric Acid Production - Mass and Energy Balances
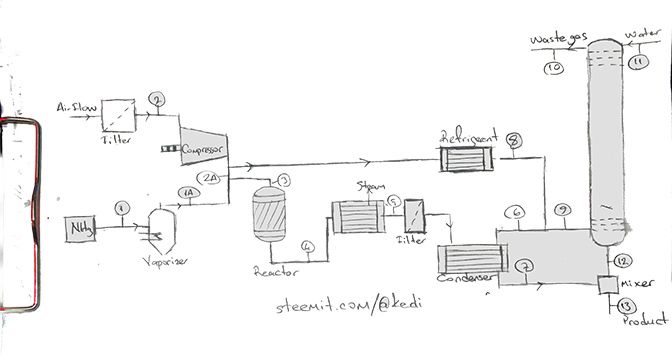
The purpose of this process is to produce nitric acid by anhydrous ammonia. Drawing of the flow chart for the process and mass and energy balances are required. Constitutively nitric acid production is that the nitrogen monoxide in the gases formed by the oxidation of ammonia is converted into nitric acid by absorbing it with water. Three different processes are proposed for nitric acid production at the sources. These are,
- Oxidation and absorption at atmospheric pressure
- Oxidation and absorption at high pressure (approximately 8 atm)
- Oxidation at atmospheric pressure and absorption at high pressure
In this work we will use second option.

Theoretical Knowledge

Nitric acid, which is very common in use, is a clear yellowish liquid. It contains nitrogen, hydrogen and oxygen atoms. The formula is HNO3. Nitric acid can be produced in three ways. These are, reacting the Chilean saltpeter with sulfuric acid, catalytic burning of ammonia and the burning of the air components. Catalytic burning (Ostnvald prodecure) is the most commonly used way of production nowadays. Nitric acid technical (spesific) properties are,
| Chemical Name | Nitric Acid |
|---|---|
| Formula | HNO3 |
| Molecular Weight | 63.02 g/mol |
| Color / Form | Yellowish / Liquid |
| Density | 1,339 ( % 55 ) - 1,1150 ( % 20 ) - 1,3667 ( % 60 ) g/cm³ |
| Freezing Point | 17 ° C ( % 20 ) - 22,4 ° C ( % 60 ) |
| Boiling Point | 103,4 °C ( % 20 ) - 120,4 °C ( % 60 ) |
Areas of Usage - Nitric acid is used in fertilizer production, metal industry, paint industry, explosive materials industry, pH balancing, electropolishing etc.
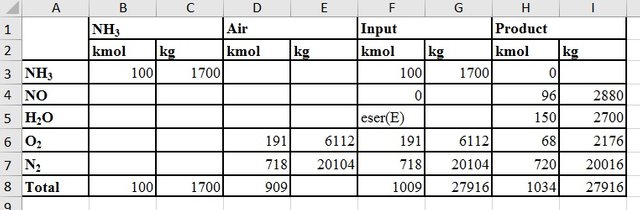
Mass Balances for The Production of Nitric Acid
Let’s suppose that,
- Annual operating time is 8000 hours
- Process yield is %94 over ammonia
- Reactor yield is %96
- The nitric acid concentration to be produced is 58% by weight
- There is 0.2% NO by volume in the exhaust gases
- The risk of explosion may be high if the ammonia being transported is more than 12%. The concentration of ammonia in the inlet should be above 11%
As a basis for mass balances, ammonia entering the reactor is taken as 100 kmol.
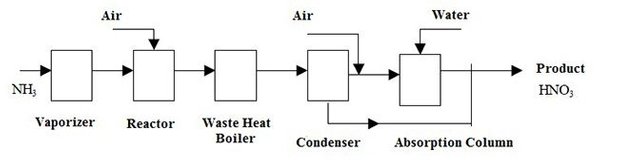
Oxidation Unit

The yield of the reaction no 1 that by %96,
NH3(g) + 5/4 O2(g) -» NO(g) + 3/2 H2O(g)
| Formed NO | 100*(96/100) = 96 kmol |
|---|---|
| Required Oxygen | 96x(5/4) = 120 kmol |
| Formed water | 96x(3/2) = 144 kmol |
4% of the ammonia is consumed in the input for nitrogen by reaction No. 2 formation.
NH3(g) + 3/4 O2(g) --» 1/2 N2(g) + 3/2 H2O(g)
| Amount of nitrogen produced | 4/2 = 2 kmol |
|---|---|
| Amount of oxygen required | 2x3/2 = 3 kmol |
| Amount of water produced | 3x2 = 6 kmol |
| Total amount of water produced | 144 + 6 = 150 kmol |
| Stoichiometric amount of oxygen | 120 + 3 = 123 kmol |
NO oxidises to NO2 by courtesty of the excess air in the input
NO(g) + 12 O2(g) --» NO2(g)
If the input ammonia concentration is taken as 11% by volume,
| Amount of air in the inlet | 100x(100/11) = 909 kmol |
|---|---|
| Amount of oxygen | 909x(79/100) = 718 kmol |
| Amount of oxygen that does not react | 191 - 123 = 68 kmol |
| Amount of nitrogen in the product stream | 718 + 2 = 720 kmol |
The amounts of the currents in the oxidation unit:
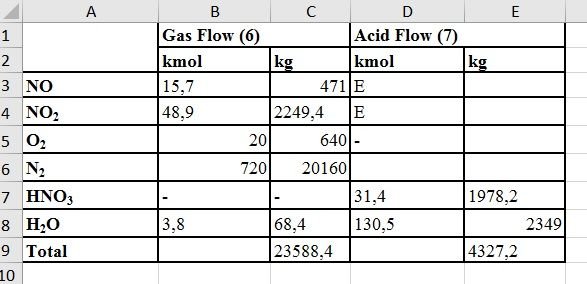
Mass Balance for The Condenser

- 45% of the stream exiting the condenser is nitric acid
- Let's basically assume that there is 100 kmol HNO3 in the liquid stream (condensate) leaving the condenser
3NO2 + H2O --» 2HNO3 + NO| Amount of water required to form 100 kmol HNO3 | 50 kmol = 900 kg |
|---|---|
| Mass of 100 kmol HNO3 | 100x63 = 630 kg |
| Amount of water required to dilute 630 kg acid as 45% | (6300x55) / 45 = 7700 kg |
| Total amount of water required to obtain dilute acid | 900 + 7700 = 8600 kg |
| Formed HNO3 | (Amount of water preduced per 100 kmoles of NH3 in the inlet) / ( Amount of water required to make 100 kmol of HNO3 to 45% solution) = 100x(2700/8600) = 31,4 kmol |
| Amount of NO2 consumed in the No.5 reaction | 31,4x(3/2) = 47,1 kmol |
| Amount of NO reacted | 31,4x(1+2) = 15,7 kmol |
| Amount of water reacted | 15,7 kmol |
The amount of water that is condensing but not reacting with NO2 = 150 -15,7 = 134,3 kmol
The amount of NO in the gas stream from the condenser can be equal to the amount of NO2 that forms HNO3 by absorbing it in the condensate. Therefore, the amount of NO in the gas stream from the condenser = 15.7 kmol
Mass Balance for Nitrogen Oxides
The total amount of NO + NO2 entering the unit = No.4 = 96 kmol
31.4 kmol , leaves the unit as HNO3. For this reason, the total amount of NO + NO2 in the gas stream = 96 -31,4 = 64,6 kmol
Assuming this is 15.7 kmol of NO, the amount of NO2 in the outgoing gas stream = 64,6-15,7 = 48,9 kmol
Mass Balance for Oxygen
Let’s suppose that,
The amount of oxygen not reacted is x moles
The amount of oxygen resulting from the unit = The amount of oxygen in stream 6 + The amount of oxygen in stream 4
= [NO/2 + NO2 + x ] + [3/2 *HNO3 +H2O/2] = (171 + x) kmol
= [15,7/2 + 48,9 + x ] + [3/2 *31,4 +134,3/2] = (171 + x) kmol
Amount of oxygen entering the unit = Amount of oxygen in stream 5
= [NO/2 + O2 + H2O] = [96/2 + 68 + 150/2] = 191 kmol191= 171 + x x=20kmol (it is the amount leaving the unit without entering the reaction)
To calculate how much of the water vapor in the condenser input stream is concentrated and how much remains in the vapor phase, as a preliminary experiment, suppose that the entire water vapor is concentrated.
| Amount of water in the condenser leaving stream | Water mole fraction x Total flow rate |
|---|---|
| Total flow rate of this stream (except water vapor) | 804,6 kmol |
| The mole fraction in this stream of water | 4,77x10^3 |
| Amount of water vapor | 4,77x10^3x804,6 = 3,8 kmol |
| The condensed water vapor content | 134,3 - 3,8 = 130,5 kmol |
| Amount of water in stream 6 | 3,8 kmol = 68,4 kg |
| Amount of water in stream 7 | 134,3 - 3,8 = 130,5 kmol = 2349 kg |
Absorption Unit
Let’s suppose that,
NO2 in the gas stream entering the absorption unit is absorbed by water and a %60 by weight nitric acid solution is formed. The amount of oxygen in the incoming stream should be the amount that will increase NO to NO2. The amount of oxygen in the waste gases leaving the absorption unit should be kept at around %3
By taking advantage of the combination of 6 currents, the amount of NO in the current entering the unit = 15.7 kmol of oxygen = 20 kmol
The amount of oxygen needed to oxidize NO2 in the input stream = 15,7 / 2 = 7,85 kmol
The amount of free oxygen in the incoming stream = 20 -7,85 = 12,15 kmol
Amount of oxygen required = (48,9 + 15,7)x(1/4) = 16,15 kmol
16,15-12,15 = 4 mol oxygen should be sent
If y mol of secondary air stream is sent to the absorption unit, 0.21 kmol of oxygen will be in this stream. This oxygen will be consumed for 4 kmol NO oxidation and the amount of oxygen in the waste gases will be 0,21y - 4 kmol.
Nitrogen will not undergo any change in the absorption unit. Therefore, amount of nitrogen in exhaust gases; equal to the sum of the amount of nitrogen in the stream coming from the condenser to the absorption unit plus the amount of nitrogen in the secondary air.
The amount of nitrogen in the waste gas = 720 + 0,79y kmol
If we assume that there is only oxygen and nitrogen in the flue gas stream, and that the amount of other gases is negligible, the percentage of oxygen in this stream is %3. y = 141,6 kmol
The amount of oxygen in the waste gas stream = (141,6x0,21) - 4 = 25,7 kmol
The amount of nitrogen in the waste gas stream = (141,6x0,79)+ 720 = 831,8 kmol
If we assume that the amount of NO in the waste gas stream is 0.2%, the amount of NO in the waste gas stream = total flow rate x 0,002 = (831,8 + 25,7)x0,002 = 1,7 kmol
The amount of oxygen in the waste gas stream = 25,7 + 1,7x(1/4 + 1/2) = 27,0 kmol
Absorbed nitrogen oxides = 48,9 +15,7) - 1,7 = 62,9 kmol = 3962,7 kg
Using the 6-way reaction method, the necessary stoichiometric amount of water = (62,9/4)x2 = 31,5 kmol
If the concentration of acid stream from the absorption unit is 60% by weight, amount of water required for dilution = = (3962,7 / 0,6)x0,4 = 2641,8 kg = 146,8 kmol
The amount of acid formed is based on the reaction of 6,
31,5 kmol H2O x 4kmolHNO3/2kmolH2O = 63 kmol HNO3
Energy Balances of Nitric Acid Production
Ammonia Vaporizer
Ammonia will be collected under pressure as liquid. The saturation temperature at 8 atm is 20 °C. Let's assume that the feed is done at 15 ° C, at the evaporator ambient temperature.
| Specific heat at 8 bar | 4,5 kJ/kgK |
|---|---|
| Covert heat at 8 bar | 1186 kJ/kg |
| Vaporizer inlet velocity | 731 kg/st |
| The inlet temperature required to raise the temperature to 20 ° C and evaporate; | 731x[4,5x(20-15) + 1186] = 883413,5 kJ/st |
| With the addition of 10% excess for heat losses | 1,1x883413,5 = 971754,9 kJ/st ≈972 MJ/st |
Mixing Point
| For Air | --------- |
|---|---|
| Feed rate | 11272,9 kg/st |
| Feed temperature | 230 °C |
| Cp Air | 1 kJ/kgK |
| For NH3 Vapor | --------- |
|---|---|
| Feed rate | 731 kg/st |
| Feed temperature | 20 °C |
| Cp NH3 Vapor | 2,2 kJ/kgK |
The energy balance around the confluence point,
11272,9x1(230 - t3 )+731x2,2x(20 - t3) = 0
t3 = 204 °C
Energy Balance in The Absorption Tower
Heat source in the absorption column will be the same as the condenser and the same calculation method will be used.
| Tower | --------- |
|---|---|
| Heat in the secondary air | 1754,8x1(40-25) = 0,018 GJ/st |
| Heat in tail gases (at 25 ° C) | 0 °C |
| Heat in the feed water (at 25 ° C) | 0 °C |
| Oxidized NO | (202,5-21,9)/30 = 6,02 kmol/st |
| Produced heat | 6,02x57120 = 0,34 GJ/st |
| Formed HNO3 | 1704/63 = 27,05 kmol/st |
| Produced heat 2 | 27,05x63640 = 1,72 GJ/st |
| Dilution temperature of %60 at 25 ° C | 27,05x14207 = 0,38 GJ/st |
| Condensed water | 29,4 - 26,3 = 3,1 kg/st |
| Latent heat at 40 ° C | 2405 kJ/st |
| Heat at reference temperature | 4,18x(40 - 25) = 63 kJ/kg |
| Heat released out | 3,1x(2405 + 63) = 7,6x10-3 GJ/st |
| Heat in the ascending acid | 0,64x(40 - 25 ) = 0,11 GJ/st |
References:
- Keleti, C. (1085), Nitric Acid and Fertilizer Nitrates ( Fertilizer Science & Technology Series), CRC Press
- http://www.essentialchemicalindustry.org/chemicals/nitric-acid.html
- R.M. Harrison and H.A. McCartney, (1979) Some Measurements of Ambient Air Pollution Arising from the Manufacture of Nitric Acid and Ammonium Nitrate Fertilizer, Atmos
I don't know why, but that post reminds me the movie Fight Club.
Makale çok güzel teşekkürler
Can you please elaborate the above step, how you used 6300 kg instead of 630 kg?
If we assume that there is only oxygen and nitrogen in the flue gas stream, and that the amount of other gases is negligible, the percentage of oxygen in this stream is %3. y = 141,6 kmol. How do you get 141,6 kmol? From what stream do you get 3% of?