Actually, The "DIAMOND" Was a Charcoal (Carbon)
Carbon
Carbon
 Source :kahnsjewellers
Source :kahnsjewellers
Diamond is a precious item made for jewelry, it has a high selling price around $514 USD (1/3 Carat), In the science version the diamond is a shaped crystal of chemical mineral or carbon allotrope. Diamond has special physical properties, especially its hardness and its ability to disperse the light, this mater make the diamonds used in jewelry and various applications in word industry. Many people think the diamond was a rare stuff, therefore I will explain about diamond and the composer of diamond in chemistry. Chemically, The diamond is formed from allotrope carbon, what is allotrope carbon? Allotrope Carbon (C) is a mineral compound formed by Carbon (C) elements with different structures. Examples of allotropes are Graphite, Diamond, Amorphous Carbon, and Fullerene.
Diamond Source
 Source :atlasofthefuture
Source :atlasofthefuture
Carbon (C) is an abundant element in the earth's crust with abundance concentration about 1800 ppm (wikipedia), However, the carbon in the earth crust was bound to other compounds such as Calcium Carbonate (CaCO3), Carbon Dioxide (CO2), and various of organic compounds. In addition, the carbon does found also as free element in graphite form (charcoal) and diamond. The diamonds and graphite are composed of same elements but have different structures, in diamond compound the single carbon atom (C) binds covalently to four (4) of atom carbon and form to tetrahedral, whereas graphite compounds each atom C bound to three (3) of other carbon atom and form to hexagonal arrangement. Because electron of atom (C) on graphite was bound weakly by atomic nucleus, so the electrons can flow from one atom to another atom, so that the graphite can transmit electricity. Therefore, the graphite was widely used as electrode in battery and electrolysis cells, Except of transmitting electricity, graphite also slippery used for lubricants.
The carbon produced from wood burning and other organic matter is called as charcoal, if charcoal heated at 800°C it will become as active charcoal (carbon active) that used in the sugar bleaching, water purification as absorber for other substances, if the carbon produced from hydrocarbon combustion called as carbon black that used in rubber vulcanization in the tire industry and black pigment (dyes) in the paint and paper ink. Diamond is a hard sparkling mineral, every atom C bound to 4 other atoms then form tetrahedron structure, this structure continues to form strong network. By technology, the graphite compounds can be transformed into synthetic diamonds at high pressure and temperatures, Due to its hardness, the synthetic diamonds used for glass cutter and drill.
Natural Diamond
 Source :pinterest
Source :pinterest
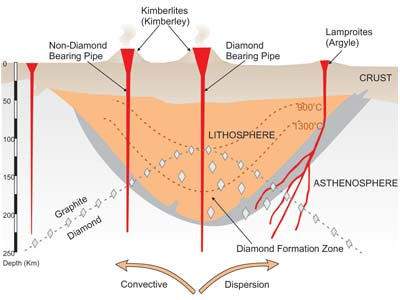
Naturally, the diamond process formation through a magmatism process that binds to the earth surface, this matter make kimberlite channel which containing carbon that turn the carbon into diamonds at high temperatures over certain time period. The diamond is not a genuine mineral, as they were formed at high temperature and pressure on the arms mantel, about 100 miles under surface. In fact, the most of diamonds found on the earth surface are the result of submissions from under surface by volcanic eruptions. The volcano eruption begin from magma journey from the mantle contain the diamond which break the rocky mantel and drove it to the surface without melting.
The stone mantel is known as xenolith, it contains diamonds formed at high pressure and temperatures of the mantel, most of mining diamonds contain xenolith rocks and alluvial sediments which are weathering result of rock blocks containing diamonds that have been transported by water media.
Synthetic Diamond
 Source :proprofs
Source :proprofs
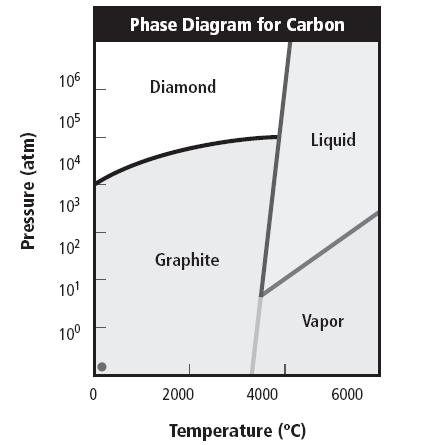
In the laboratory the diamond produce commercially with graphite and metal catalysts, the process of diamond making by using high temperature about 3500°C or higher and pressure about 140,000 atm. Additionally, the diamonds can also produced using hydrocarbons pyrolysis at relative low temperatures about 900°C and low pressure around 102 Pa. However, in the synthesis process, the diamond has 3 different colors, the resulting color was influenced by several things like impurities content, in the diamond structure there is a chemical pollutant that can alter the absorption spectrum of diamonds, the spectrum changes due to the impurities, this color result depends of impurities kind and its concentration, Such as, yellow and orange diamonds contain Nitrogen (N2), Blue diamonds contain boron (B), and purple, green and gray diamond are contain hydrogen (H2).
The natural radiation can transform diamonds into green color, which happened over millions years so that it can alter the structure of diamond atom. Cause of structural changes the absorption spectrum also will be change. And the plastic deformation of diamond can change the diamonds color to red, pink, and brown. The change of carbon structure that rotating during diamond forming in the soil, so it change the spectrum absorption, the spectrum can be seen on diamonds as a line resembling a veins, this line make different spectrum color.
Conclusion
Conclusion
Diamond and graphite are actually have same elements, because different of atom structure so they have different looks, the form of carbon structure is also affected by pressure and temperature, so they have different violence levels, in diamond the carbon bonds in tetrahedron form while graphite form on hexagonal. Therefore, the diamond price more expensive then graphite, if we examine diamond prices are more expensive because it formed at high pressure and temperature so the atomic chain of carbon becomes covalent.
Source :
Support Scientist By Use #science tag or join @steemSTEM
Follow Me @jamhuery

Congratulations! This post has been upvoted from the communal account, @minnowsupport, by jamhuery from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews/crimsonclad, and netuoso. The goal is to help Steemit grow by supporting Minnows and creating a social network. Please find us in the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.